Interstrand DNA cross-links induced by alpha,beta-unsaturated aldehydes derived from lipid peroxidation and environmental sources
- PMID: 18500830
- PMCID: PMC2785109
- DOI: 10.1021/ar700246x
Interstrand DNA cross-links induced by alpha,beta-unsaturated aldehydes derived from lipid peroxidation and environmental sources
Abstract
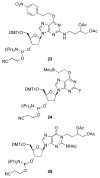
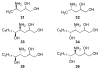
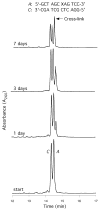
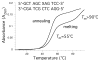
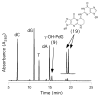
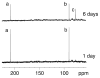
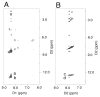
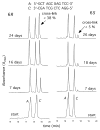
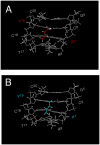
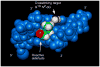
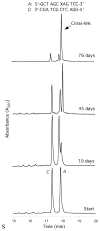
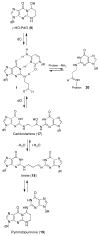
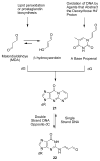
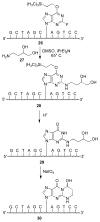
Significant levels of the 1, N(2)-gamma-hydroxypropano-dG adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxy-2E-nonenal (HNE) have been identified in human DNA, arising from both exogenous and endogenous exposures. They yield interstrand DNA cross-links between guanines in the neighboring C.G and G.C base pairs located in 5'-CpG-3' sequences, as a result of opening of the 1,N(2)-gamma-hydroxypropano-dG adducts to form reactive aldehydes that are positioned within the minor groove of duplex DNA. Using a combination of chemical, spectroscopic, and computational methods, we have elucidated the chemistry of cross-link formation in duplex DNA. NMR spectroscopy revealed that, at equilibrium, the acrolein and crotonaldehyde cross-links consist primarily of interstrand carbinolamine linkages between the exocyclic amines of the two guanines located in the neighboring C.G and G.C base pairs located in 5'-CpG-3' sequences, that maintain the Watson-Crick hydrogen bonding of the cross-linked base pairs. The ability of crotonaldehyde and HNE to form interstrand cross-links depends upon their common relative stereochemistry at the C6 position of the 1,N(2)-gamma-hydroxypropano-dG adduct. The stereochemistry at this center modulates the orientation of the reactive aldehyde within the minor groove of the double-stranded DNA, either facilitating or hindering the cross-linking reactions; it also affects the stabilities of the resulting diastereoisomeric cross-links. The presence of these cross-links in vivo is anticipated to interfere with DNA replication and transcription, thereby contributing to the etiology of human disease. Reduced derivatives of these cross-links are useful tools for studying their biological processing.
Figures
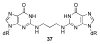



References
-
- Burcham PC. Genotoxic lipid peroxidation products: Their DNA damaging properties and role in formation of endogenous DNA adducts. Mutagenesis. 1998;13:287–305. - PubMed
-
- Chung FL, Nath RG, Nagao M, Nishikawa A, Zhou GD, Randerath K. Endogenous formation and significance of 1,N2-propanodeoxyguanosine adducts. Mutat Res. 1999;424:71–81. - PubMed
-
- Chung FL, Zhang L, Ocando JE, Nath RG. Role of 1,N2-propanodeoxyguanosine adducts as endogenous DNA lesions in rodents and humans. IARC Sci Pub. 1999;150:45–54. - PubMed
-
- Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: A review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43:1109–1120. - PubMed
-
- Hecht SS, Upadhyaya P, Wang M. Reactions of α-acetoxy-N-nitrosopyrrolidine and crotonaldehyde with DNA. IARC Sci Pub. 1999;150:147–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

