Cloning, characterization, and expression of microRNAs from the Asian malaria mosquito, Anopheles stephensi
- PMID: 18500992
- PMCID: PMC2430712
- DOI: 10.1186/1471-2164-9-244
Cloning, characterization, and expression of microRNAs from the Asian malaria mosquito, Anopheles stephensi
Abstract
Background: microRNAs (miRNAs) are non-coding RNAs that are now recognized as a major class of gene-regulating molecules widely distributed in metozoans and plants. miRNAs have been found to play important roles in apoptosis, cancer, development, differentiation, inflammation, longevity, and viral infection. There are a few reports describing miRNAs in the African malaria mosquito, Anopheles gambiae, on the basis of similarity to known miRNAs from other species. An. stephensi is the most important malaria vector in Asia and it is becoming a model Anopheline species for physiological and genetics studies.
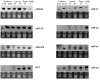
Results: We report the cloning and characterization of 27 distinct miRNAs from 17-day old An. stephensi female mosquitoes. Seventeen of the 27 miRNAs matched previously predicted An. gambiae miRNAs, offering the first experimental verification of miRNAs from mosquito species. Ten of the 27 are miRNAs previously unknown to mosquitoes, four of which did not match any known miRNAs in any organism. Twenty-five of the 27 Anopheles miRNAs had conserved sequences in the genome of a divergent relative, the yellow fever mosquito Aedes aegypti. Two clusters of miRNAs were found within introns of orthologous genes in An. gambiae, Ae. aegypti, and Drosophila melanogaster. Mature miRNAs were detected in An. stephensi for all of the nine selected miRNAs, including the four novel miRNAs (miR-x1- miR-x4), either by northern blot or by Ribonuclease Protection Assay. Expression profile analysis of eight of these miRNAs revealed distinct expression patterns from early embryo to adult stages in An. stephensi. In both An. stephensi and Ae. aegypti, the expression of miR-x2 was restricted to adult females and predominantly in the ovaries. A significant reduction of miR-x2 level was observed 72 hrs after a blood meal. Thus miR-x2 is likely involved in female reproduction and its function may be conserved among divergent mosquitoes. A mosquito homolog of miR-14, a regulator of longevity and apoptosis in D. melanogaster, represented 25% of all sequenced miRNA clones from 17-day old An. stephensi female mosquitoes. An. stephensi miR-14 displayed a relatively strong signal from late embryonic to adult stages. miR-14 expression is consistent during the adult lifespan regardless of age, sex, and blood feeding status. Thus miR-14 is likely important across all mosquito life stages.
Conclusion: This study provides experimental evidence for 23 conserved and four new microRNAs in An. stephensi mosquitoes. Comparisons between miRNA gene clusters in Anopheles and Aedes mosquitoes, and in D. melanogaster suggest the loss or significant change of two miRNA genes in Ae. aegypti. Expression profile analysis of eight miRNAs, including the four new miRNAs, revealed distinct patterns from early embryo to adult stages in An. stephensi. Further analysis showed that miR-x2 is likely involved in female reproduction and its function may be conserved among divergent mosquitoes. Consistent expression of miR-14 suggests that it is likely important across all mosquito life stages from embryos to aged adults. Understanding the functions of mosquito miRNAs will undoubtedly contribute to a better understanding of mosquito biology including longevity, reproduction, and mosquito-pathogen interactions, which are important to disease transmission.
Figures



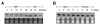

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

