Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury
- PMID: 18501200
- PMCID: PMC2586827
- DOI: 10.1016/j.freeradbiomed.2008.04.038
Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury
Abstract
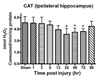
Oxidative stress, an imbalance between oxidants and antioxidants, contributes to the pathogenesis of traumatic brain injury (TBI). Oxidative neurodegeneration is a key mediator of exacerbated morphological responses and deficits in behavioral recoveries. The present study assessed early hippocampal sequential imbalance to possibly enhance antioxidant therapy. Young adult male Sprague-Dawley rats were subjected to a unilateral moderate cortical contusion. At various times post-TBI, animals were killed and the hippocampus was analyzed for antioxidants (GSH, GSSG, glutathione peroxidase, glutathione reductase, glutathione-S-transferase, glucose-6-phosphate dehydrogenase, superoxide dismutase, and catalase) and oxidants (acrolein, 4-hydroxynonenal, protein carbonyl, and 3-nitrotyrosine). Synaptic markers (synapsin I, postsynaptic density protein 95, synapse-associated protein 97, growth-associated protein 43) were also analyzed. All values were compared with those for sham-operated animals. Significant time-dependent changes in antioxidants were observed as early as 3 h posttrauma and paralleled increases in oxidants (4-hydroxynonenal, acrolein, and protein carbonyl), with peak values obtained at 24-48 h. Time-dependent changes in synaptic proteins (synapsin I, postsynaptic density protein 95, and synapse-associated protein 97) occurred well after levels of oxidants peaked. These results indicate that depletion of antioxidant systems following trauma could adversely affect synaptic function and plasticity. Early onset of oxidative stress suggests that the initial therapeutic window following TBI appears to be relatively short, and it may be necessary to stagger selective types of antioxidant therapy to target specific oxidative components.
Figures








References
-
- Sullivan PG, Keller JN, Mattson MP, Scheff SW. Traumatic brain injury alters synaptic homeostasis: implications for impaired mitochondrial and transport function. J. Neurotrauma. 1998;15:789–798. - PubMed
-
- Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, Yang Y, Awasthi S, Awasthi YC, Zimniak P. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: generation and analysis of mGsta4 null mouse. Toxicol. Appl. Pharmacol. 2004;194:296–308. - PubMed
-
- Shao C, Roberts KN, Markesbery WR, Scheff SW, Lovell MA. Oxidative stress in head trauma in aging. Free Radic. Biol. Med. 2006;41:77–85. - PubMed
-
- Ozdemir D, Uysal N, Gonenc S, Acikgoz O, Sonmez A, Topcu A, Ozdemir N, Duman M, Semin I, Ozkan H. Effect of melatonin on brain oxidative damage induced by traumatic brain injury in immature rats. Physiol. Res. 2005;54:631–637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

