Deoxynivalenol induces p38 interaction with the ribosome in monocytes and macrophages
- PMID: 18502741
- PMCID: PMC6592419
- DOI: 10.1093/toxsci/kfn102
Deoxynivalenol induces p38 interaction with the ribosome in monocytes and macrophages
Abstract
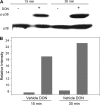
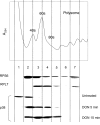
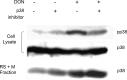
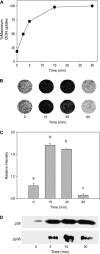
Trichothecene mycotoxins rapidly induce p38-mediated gene expression and apoptosis in mononuclear phagocytes via a process known as the ribotoxic stress response. We hypothesized that the trichothecene deoxynivalenol (DON) induces interaction of p38 with the ribosome. Two models, U937 human monocytes and RAW 264.7 murine macrophages, were used to test this hypothesis based on their capacity to evoke rapid and robust p38 phosphorylation responses to DON. Following DON treatment of U937 cells, lysates were subjected to sucrose gradient fractionation and the resultant ribosomal fractions probed for p38 by Western blotting. p38 content in fractions containing ribosomal subunits and monosomes (RS + M) increased within 5 min of DON treatment and continued to increase up to 30 min. p38 appeared to be initially interact with the 40S subunit fraction and then subsequently with the 60S unit and monosome fractions. Although p38 phosphorylation was blocked by the inhibitor SB203580, interaction of the kinase with the ribosome was unaffected, suggesting that ribosomal binding and phosphorylation were dissociable events. In RAW 264.7 cells, radiolabeled DON uptake occurred within 15 min and this corresponded to sequential increases nonphosphorylated p38 and phosphorylated p38 in the RS + M fraction. As observed for p38, DON similarly induced both ribosomal interaction with two mitogen-activated protein kinases, c-Jun N-terminal kinase, and extracellular signal-regulated kinase, and their subsequent phosphorylation in RAW 264.7 cells. Taken together, these data suggest that, in mononuclear phagocytes, DON induced p38 mobilization to the ribosome and its subsequent phosphorylation. The ribosome might thus play a central role as a scaffold in the ribotoxic stress response.
Figures




References
-
- Alisi A, Spaziani A, Anticoli S, Ghidinelli M, Balsano C. PKR is a novel functional direct player that coordinates skeletal muscle differentiation via p38MAPK/AKT pathways. Cell Signal. 2008;20:534–542. - PubMed
-
- Bunyard P, Handley M, Pollara G, Rutault K, Wood I, Chaudry M, Alderman C, Foreman J, Katz DR, Chain BM. Ribotoxic stress activates p38 and JNK kinases and modulates the antigen-presenting activity of dendritic cells. Mol. Immunol. 2003;39:815–827. - PubMed
-
- Chen-Chih WR, Shaio MF, Cho WL. A p38 MAP kinase regulates the expression of the Aedes aegypti defensin gene in mosquito cells. Insect Mol. Biol. 2007;16:389–399. - PubMed
-
- Cherla RP, Lee SY, Mees PL, Tesh VL. Shiga toxin 1-induced cytokine production is mediated by MAP kinase pathways and translation initiation factor eIF4E in the macrophage-like THP-1 cell line. J. Leukoc. Biol. 2006;79:397–407. - PubMed
-
- Chung YJ, Zhou HR, Pestka JJ. Transcriptional and posttranscriptional roles for p38 mitogen-activated protein kinase in upregulation of TNF-alpha expression by deoxynivalenol (vomitoxin) Toxicol. Appl. Pharmacol. 2003;193:188–201. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

