Microtubule assembly of isotypically purified tubulin and its mixtures
- PMID: 18502790
- PMCID: PMC2483739
- DOI: 10.1529/biophysj.108.132233
Microtubule assembly of isotypically purified tubulin and its mixtures
Abstract
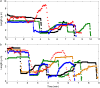
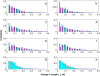
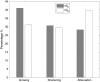
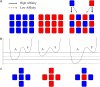
Numerous isotypes of the structural protein tubulin have now been characterized in various organisms and their expression offers a plausible explanation for observed differences affecting microtubule function in vivo. While this is an attractive hypothesis, there are only a handful of studies demonstrating a direct influence of tubulin isotype composition on the dynamic properties of microtubules. Here, we present the results of experimental assays on the assembly of microtubules from bovine brain tubulin using purified isotypes at various controlled relative concentrations. A novel data analysis is developed using recursive maps which are shown to be related to the master equation formalism. We have found striking similarities between the three isotypes of bovine tubulin studied in regard to their dynamic instability properties, except for subtle differences in their catastrophe frequencies. When mixtures of tubulin isotypes are analyzed, their nonlinear concentration dependence is modeled and interpreted in terms of lower affinities of tubulin dimers belonging to the same isotype than those that represent different isotypes indicating hitherto unsuspected influences of tubulin dimers on each other within a microtubule. Finally, we investigate the fluctuations in microtubule assembly and disassembly rates and conclude that the inherent rate variability may signify differences in the guanosine-5'-triphosphate composition of the growing and shortening microtubule tips. It is the main objective of this article to develop a quantitative model of tubulin polymerization for individual isotypes and their mixtures. The possible biological significance of the observed differences is addressed.
Figures




References
-
- Dustin, P. 1984. Microtubules, 2nd Ed. Springer, Berlin.
-
- Alberts, B., J. Lewis, M. Raff, K. Roberts, and J. D. Watson. 1994. Molecular Biology of the Cell. Garland, New York.
-
- Mitchison, T., and M. Krischner. 1984. Microtubule assembly nucleated by isolated centrosomes. Nature. 312:232–236. - PubMed
-
- Rezania, V., and J. A. Tuszynski. 2007. From a quantum mechanical description of the assembly processes in microtubules to their semiclassical nonlinear dynamics. Quantum Biosys. 1:1–20.
-
- Erickson, H. P., and T. E. O'Brien. 1992. Microtubule dynamic instability and GTP hydrolysis. Annu. Rev. Biophys. Biomol. Struct. 21:145–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

