Formate as the main branch point for methylotrophic metabolism in Methylobacterium extorquens AM1
- PMID: 18502865
- PMCID: PMC2447001
- DOI: 10.1128/JB.00228-08
Formate as the main branch point for methylotrophic metabolism in Methylobacterium extorquens AM1
Abstract
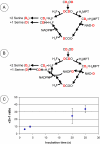
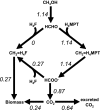
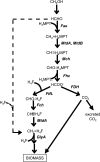
In serine cycle methylotrophs, methylene tetrahydrofolate (H4F) is the entry point of reduced one-carbon compounds into the serine cycle for carbon assimilation during methylotrophic metabolism. In these bacteria, two routes are possible for generating methylene H4F from formaldehyde during methylotrophic growth: one involving the reaction of formaldehyde with H4F to generate methylene H4F and the other involving conversion of formaldehyde to formate via methylene tetrahydromethanopterin-dependent enzymes and conversion of formate to methylene H4F via H4F-dependent enzymes. Evidence has suggested that the direct condensation reaction is the main source of methylene H4F during methylotrophic metabolism. However, mutants lacking enzymes that interconvert methylene H4F and formate are unable to grow on methanol, suggesting that this route for methylene H4F synthesis should have a significant role in biomass production during methylotrophic metabolism. This problem was investigated in Methylobacterium extorquens AM1. Evidence was obtained suggesting that the existing deuterium assay might overestimate the flux through the direct condensation reaction. To test this possibility, it was shown that only minor assimilation into biomass occurred in mutants lacking the methylene H4F synthesis pathway through formate. These results suggested that the methylene H4F synthesis pathway through formate dominates assimilatory flux. A revised kinetic model was used to validate this possibility, showing that physiologically plausible parameters in this model can account for the metabolic fluxes observed in vivo. These results all support the suggestion that formate, not formaldehyde, is the main branch point for methylotrophic metabolism in M. extorquens AM1.
Figures

References
-
- Anthony, C. 1982. The biochemistry of methylotrophs. Academic Press, New York, NY.
-
- Chistoserdova, L., G. J. Crowther, J. A. Vorholt, E. Skovran, J.-C. Portais, and M. E. Lidstrom. 2007. Identification of a fourth formate dehydrogenase in Methylobacterium extorquens AM1 and confirmation of the essential role of formate oxidation in methylotrophy. J. Bacteriol. 1899076-9081. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

