Listeria monocytogenes EGD-e biofilms: no mushrooms but a network of knitted chains
- PMID: 18502930
- PMCID: PMC2493181
- DOI: 10.1128/AEM.00255-08
Listeria monocytogenes EGD-e biofilms: no mushrooms but a network of knitted chains
Abstract
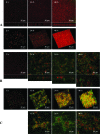
Listeria monocytogenes is a food pathogen that can attach on most of the surfaces encountered in the food industry. Biofilms are three-dimensional microbial structures that facilitate the persistence of pathogens on surfaces, their resistance toward antimicrobials, and the final contamination of processed goods. So far, little is known about the structural dynamics of L. monocytogenes biofilm formation and its regulation. The aims of this study were, by combining genetics and time-lapse laser-scanning confocal microscopy (LSCM), (i) to characterize the structural dynamics of L. monocytogenes EGD-e sessile growth in two nutritional environments (with or without a nutrient flow), and (ii) to evaluate the possible role of the L. monocytogenes agr system during biofilm formation by tracking the spatiotemporal fluorescence expression of a green fluorescent protein (GFP) reporter system. In the absence of nutrient flow (static conditions), unstructured biofilms composed of a few layers of cells that covered the substratum were observed. In contrast, when grown under dynamic conditions, L. monocytogenes EGD-e biofilms were highly organized. Indeed, ball-shaped microcolonies were surrounded by a network of knitted chains. The spatiotemporal tracking of fluorescence emitted by the GFP reporter system revealed that agr expression was barely detectable under static conditions, but it progressively increased during 40 h under dynamic conditions. Moreover, spatial analysis revealed that agr was expressed preferentially in cells located outside the microcolonies. Finally, the in-frame deletion of agrA, which encodes a transcriptional regulator, resulted in a decrease in initial adherence without affecting the subsequent biofilm development.
Figures


References
-
- Beresford, M. R., P. W. Andrew, and G. Shama. 2001. Listeria monocytogenes adheres to many materials found in food-processing environments. J. Appl. Microbiol. 90:1000-1005. - PubMed
-
- Blackman, I. C., and J. F. Frank. 1996. Growth of Listeria monocytogenes as a biofilm an various food-processing surfaces. J. Food Prot. 59:827-831. - PubMed
-
- Bower, C. K., J. McGuire, and M. A. Daeschel. 1996. The adhesion and detachment of bacteria and spores on food-contact surfaces. Trends Food Sci. Technol. 71:152-157.
-
- Brackett, R. E. 1992. Shelf stability and safety of fresh produce as influenced by sanitation and disinfection. J. Food Prot. 55:808-814. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

