Natural selection against protein aggregation on self-interacting and essential proteins in yeast, fly, and worm
- PMID: 18503047
- PMCID: PMC2727382
- DOI: 10.1093/molbev/msn122
Natural selection against protein aggregation on self-interacting and essential proteins in yeast, fly, and worm
Abstract
Protein aggregation is the phenomenon of protein self-association potentially leading to detrimental effects on physiology, which is closely related to numerous human diseases such as Alzheimer's and Parkinson's disease. Despite progress in understanding the mechanism of protein aggregation, how natural selection against protein aggregation acts on subunits of protein complexes and on proteins with different contributions to organism fitness remains largely unknown. Here, we perform a proteome-wide analysis by using an experimentally validated algorithm TANGO and utilizing sequence, interactomic and phenotype-based functional genomic data from yeast, fly, and nematode. We find that proteins that are capable of forming homooligomeric complex have lower aggregation propensity compared with proteins that do not function as homooligomer. Further, proteins that are essential to the fitness of an organism have lower aggregation propensity compared with nonessential ones. Our finding suggests that the selection force against protein aggregation acts across different hierarchies of biological system.
Figures

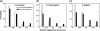

References
-
- Bastolla U, Moya A, Viguera E, van Ham RC. Genomic determinants of protein folding thermodynamics in prokaryotic organisms. J Mol Biol. 2004;343:1451–1466. - PubMed
-
- Broome BM, Hecht MH. Nature disfavors sequences of alternating polar and non-polar amino acids: implications for amyloidogenesis. J Mol Biol. 2000;296:961–968. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev of Biochem. 2006;75:333–366. - PubMed
-
- Daughdrill GW, Chadsey MS, Karlinsey JE, Hughes KT, Dahlquist FW. The C-terminal half of the anti-sigma factor, FlgM, becomes structured when bound to its target, sigma 28. Nat Struct Biol. 1997;4:285–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

