ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection
- PMID: 18503637
- PMCID: PMC2574867
- DOI: 10.1111/j.1462-5822.2008.01177.x
ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection
Abstract
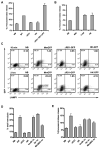
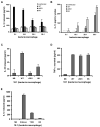
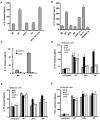
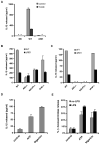
Exocytosis of lysosomes from macrophages has been described as a response to microbial cytotoxins and haemolysins, as well as for releasing pro-inflammatory cytokines interleukin (IL)-1beta and IL-18 during inflammasome activation. The mycobacterial ESX-1 secretion system, encoded in part by the Region of Difference-1, is a virulence factor necessary for phagosome escape and host cell lysis by a contact-dependent haemolysin in Mycobacterium marinum. Here we show that ESX-1 from M. marinum and M. tuberculosis is required for Ca(2+)-dependent induction of lysosome secretion from macrophages. Mycobacteria-induced lysosome secretion was concurrent to release of IL-1beta and IL-18, dependent on phagocytosis of bacteria containing ESX-1. Synthesis but not release of IL-1beta and IL-18 occurred in response to dead bacilli and bacteria lacking ESX-1, indicating that only cytokine release was regulated by ESX-1. Release of these cytokines and exocytosis of lysosomes were independent of intracellular mycobacterial growth, yet correlated with mycobacteria-encoded haemolytic activity, demonstrating a parallel pathway for the two responses. We further identified inflammasome components caspase-1, ASC and NALP3, but not Ipaf, required for release of IL-1beta and IL-18. Collectively, these results reveal a role for ESX-1 in triggering secretion of lysosomes, as well as release of IL-1beta and IL-18 during mycobacteria infection.
Figures


References
-
- Cox JS, Chen B, McNeil M, Jacobs WR., Jr Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 1999;402:79–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

