In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells
- PMID: 18504307
- PMCID: PMC2413035
- DOI: 10.1084/jem.20080034
In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells
Abstract
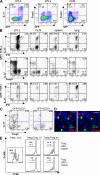
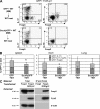
The nuclear hormone receptor retinoic acid receptor-related orphan receptor gamma t (RORgamma t) is required for the generation of T helper 17 cells expressing the proinflammatory cytokine interleukin (IL)-17. In vivo, however, less than half of RORgamma t(+) T cells express IL-17. We report here that RORgamma t(+) T alphabeta cells include Foxp3(+) cells that coexist with IL-17-producing RORgamma t(+) T alphabeta cells in all tissues examined. The Foxp3(+) RORgamma t(+) T alphabeta express IL-10 and CCL20, and function as regulatory T cells. Furthermore, the ratio of Foxp3(+) to IL-17-producing RORgamma t(+) T alphabeta cells remains remarkably constant in mice enduring infection and inflammation. This equilibrium is tuned in favor of IL-10 production by Foxp3 and CCL20, and in favor of IL-17 production by IL-6 and IL-23. In the lung and skin, the largest population of RORgamma t(+) T cells express the gammadelta T cell receptor and produce the highest levels of IL-17 independently of IL-6. Thus, potentially antagonistic proinflammatory IL-17-producing and regulatory Foxp3(+) RORgamma t(+) T cells coexist and are tightly controlled, suggesting that a perturbed equilibrium in RORgamma t(+) T cells might lead to decreased immunoreactivity or, in contrast, to pathological inflammation.
Figures





References
-
- Mosmann, T.R., and R.L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145–173. - PubMed
-
- Weaver, C.T., R.D. Hatton, P.R. Mangan, and L.E. Harrington. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25:821–852. - PubMed
-
- Kastelein, R.A., C.A. Hunter, and D.J. Cua. 2007. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25:221–242. - PubMed
-
- Nurieva, R., X.O. Yang, G. Martinez, Y. Zhang, A.D. Panopoulos, L. Ma, K. Schluns, Q. Tian, S.S. Watowich, A.M. Jetten, and C. Dong. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 448:480–483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

