Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase
- PMID: 18504457
- PMCID: PMC2742364
- DOI: 10.1038/cr.2008.61
Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase
Abstract
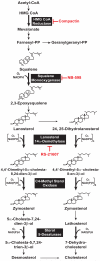
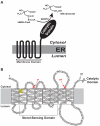
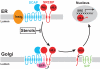
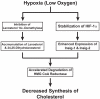
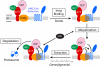
3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase produces mevalonate, an important intermediate in the synthesis of cholesterol and essential nonsterol isoprenoids. The reductase is subject to an exorbitant amount of feedback control through multiple mechanisms that are mediated by sterol and nonsterol end-products of mevalonate metabolism. Here, I will discuss recent advances that shed light on one mechanism for control of reductase, which involves rapid degradation of the enzyme. Accumulation of certain sterols triggers binding of reductase to endoplasmic reticulum (ER) membrane proteins called Insig-1 and Insig-2. Reductase-Insig binding results in recruitment of a membrane-associated ubiquitin ligase called gp78, which initiates ubiquitination of reductase. This ubiquitination is an obligatory reaction for recognition and degradation of reductase from ER membranes by cytosolic 26S proteasomes. Thus, sterol-accelerated degradation of reductase represents an example of how a general cellular process (ER-associated degradation) is used to control an important metabolic pathway (cholesterol synthesis).
Figures
References
-
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. - PubMed
-
- Brown MS, Goldstein JL. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J. Lipid Res. 1980;21:505–517. - PubMed
-
- Endo A, Kuroda M, Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976;72:323–326. - PubMed
-
- Nakanishi M, Goldstein JL, Brown MS. Multivalent control of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mevalonate-derived product inhibits translation of mRNA and accelerates degradation of enzyme. J. Biol. Chem. 1988;263:8929–8937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

