Expression of homothorax and extradenticle mRNA in the legs of the crustacean Parhyale hawaiensis: evidence for a reversal of gene expression regulation in the pancrustacean lineage
- PMID: 18504609
- PMCID: PMC2668558
- DOI: 10.1007/s00427-008-0221-4
Expression of homothorax and extradenticle mRNA in the legs of the crustacean Parhyale hawaiensis: evidence for a reversal of gene expression regulation in the pancrustacean lineage
Abstract
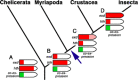
In Drosophila leg development, the extradenticle (exd) gene is expressed ubiquitously and its co-factor homothorax (hth) is restricted to the proximal leg portion. This condition is conserved in other insect species but is reversed in chelicerates and myriapods. As the region of co-expression does not differ in the two groups and transcripts from both are necessary for function, this difference in expression is likely to be functionally neutral. Here, we report the expression patterns of exd and hth in a crustacean, the amphipod shrimp Parhyale hawaiensis. The patterns in P. hawaiensis are similar to the insect patterns, supporting the close relationship between crustaceans and insects in the taxon Tetraconata. However, mRNA expression of exd in P. hawaiensis is weak in the distal leg parts, thus being intermediate between the complete lack of distal exd expression in chelicerates and myriapods and the strong distal exd expression in insects. Our data suggest that the reversal of the gene expression regulation of hth and exd occurred in the pancrustacean lineage.
Figures




Similar articles
-
Gene expression in spider appendages reveals reversal of exd/hth spatial specificity, altered leg gap gene dynamics, and suggests divergent distal morphogen signaling.Dev Biol. 2003 Dec 1;264(1):119-40. doi: 10.1016/j.ydbio.2003.08.002. Dev Biol. 2003. PMID: 14623236
-
Gene expression patterns in an onychophoran reveal that regionalization predates limb segmentation in pan-arthropods.Evol Dev. 2010 Jul-Aug;12(4):363-72. doi: 10.1111/j.1525-142X.2010.00423.x. Evol Dev. 2010. PMID: 20618432
-
Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein.Cell. 1997 Oct 17;91(2):171-83. doi: 10.1016/s0092-8674(00)80400-6. Cell. 1997. PMID: 9346235
-
A balance between two nuclear localization sequences and a nuclear export sequence governs extradenticle subcellular localization.Genetics. 2007 Apr;175(4):1625-36. doi: 10.1534/genetics.106.066449. Epub 2007 Feb 4. Genetics. 2007. PMID: 17277370 Free PMC article.
-
Non-insect crustacean models in developmental genetics including an encomium to Parhyale hawaiensis.Curr Opin Genet Dev. 2016 Aug;39:149-156. doi: 10.1016/j.gde.2016.07.004. Epub 2016 Jul 29. Curr Opin Genet Dev. 2016. PMID: 27475080 Review.
Cited by
-
High through-put sequencing of the Parhyale hawaiensis mRNAs and microRNAs to aid comparative developmental studies.PLoS One. 2012;7(3):e33784. doi: 10.1371/journal.pone.0033784. Epub 2012 Mar 20. PLoS One. 2012. PMID: 22448274 Free PMC article.
-
Rapid diversification of homothorax expression patterns after gene duplication in spiders.BMC Evol Biol. 2017 Jul 14;17(1):168. doi: 10.1186/s12862-017-1013-0. BMC Evol Biol. 2017. PMID: 28709396 Free PMC article.
-
Multi-view light-sheet imaging and tracking with the MaMuT software reveals the cell lineage of a direct developing arthropod limb.Elife. 2018 Mar 29;7:e34410. doi: 10.7554/eLife.34410. Elife. 2018. PMID: 29595475 Free PMC article.
-
Ablation of a single cell from eight-cell embryos of the amphipod crustacean Parhyale hawaiensis.J Vis Exp. 2014 Mar 16;(85):51073. doi: 10.3791/51073. J Vis Exp. 2014. PMID: 24686416 Free PMC article.
-
Knockout of crustacean leg patterning genes suggests that insect wings and body walls evolved from ancient leg segments.Nat Ecol Evol. 2020 Dec;4(12):1703-1712. doi: 10.1038/s41559-020-01349-0. Epub 2020 Dec 1. Nat Ecol Evol. 2020. PMID: 33262517
References
-
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

