Electrochemically protected copper(I)-catalyzed azide-alkyne cycloaddition
- PMID: 18504727
- PMCID: PMC3574790
- DOI: 10.1002/cbic.200700768
Electrochemically protected copper(I)-catalyzed azide-alkyne cycloaddition
Abstract
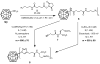
The copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction has found broad application in myriad fields. For the most demanding applications that require high yields at low substrate concentrations, highly active but air-sensitive copper complexes must be used. We describe here the use of an electrochemical potential to maintain catalysts in the active Cu(I) oxidation state in the presence of air. This simple procedure efficiently achieves excellent yields of CuAAC products from both small-molecule and protein substrates without the use of potentially damaging chemical reducing agents. A new water-soluble carboxylated version of the popular tris(benzyltriazolylmethyl)amine (TBTA) ligand is also described. Cyclic voltammetry revealed reversible or quasi-reversible electrochemical redox behavior of copper complexes of the TBTA derivative (2; E(1/2)=60 mV vs. Ag/AgCl), sulfonated bathophenanthroline (3; E(1/2)=-60 mV), and sulfonated tris(benzimidazoylmethyl)amine (4; E(1/2) approximately -70 mV), and showed catalytic turnover to be rapid relative to the voltammetry time scale. Under the influence of a -200 mV potential that was established by using a reticulated vitreous carbon working electrode, CuSO4 and 3 formed a superior catalyst. Electrochemically protected bioconjugations in air were performed by using bacteriophage Qbeta that was derivatized with azide moieties at surface lysine residues. Complete derivatization of more than 600 reactive sites per particle was demonstrated within 12 h of electrolysis with substoichiometric quantities of Cu3.
Figures
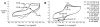

References
-
- Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004–2021. - PubMed
-
- Hawker CJ, Fokin VV, Finn MG, Sharpless KB. Aust J Chem. 2007;60:381–383.
-
- Tornøe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3062. - PubMed
-
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed. 2002;41:2596–2599. - PubMed
-
- Evans RA. Aust J Chem. 2007;60:384–395.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

