Myoblasts from affected and non-affected FSHD muscles exhibit morphological differentiation defects
- PMID: 18505476
- PMCID: PMC2910739
- DOI: 10.1111/j.1582-4934.2008.00368.x
Myoblasts from affected and non-affected FSHD muscles exhibit morphological differentiation defects
Abstract
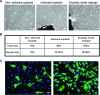
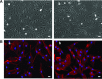
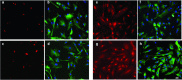
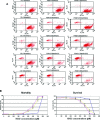
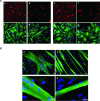
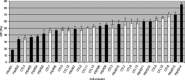
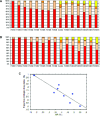
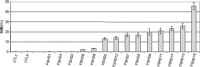
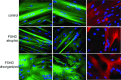
Facioscapulohumeral dystrophy (FSHD) is a muscular hereditary disease with a prevalence of 1 in 20,000 caused by a partial deletion of a subtelomeric repeat array on chromosome 4q. However, very little is known about the pathogenesis as well as the molecular and biochemical changes linked to the progressive muscle degeneration observed in these patients. Several studies have investigated possible pathophysiological pathways in FSHD myoblasts and mature muscle cells but some of these reports were apparently in contradiction. The discrepancy between these studies may be explained by differences between the sources of myoblasts. Therefore, we decided to thoroughly analyze affected and unaffected muscles from patients with FSHD in terms of vulnerability to oxidative stress, differentiation capacity and morphological abnormalities. We have established a panel of primary myoblast cell cultures from patients affected with FSHD and matched healthy individuals. Our results show that primary myoblasts are more susceptible to an induced oxidative stress than control myoblasts. Moreover, we demonstrate that both types of FSHD primary myoblasts differentiate into multi-nucleated myotubes, which present morphological abnormalities. Whereas control myoblasts fuse to form branched myotubes with aligned nuclei, FSHD myoblasts fuse to form either thin and branched myotubes with aligned nuclei or large myotubes with random nuclei distribution. In conclusion, we postulate that these abnormalities could be responsible for muscle weakness in patients with FSHD and provide an important marker for FSHD myoblasts.
Figures

References
-
- Van Deutekom JC, Wijmenga C, Van Tienhoven EA, et al. FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit. Hum Mol Genet. 1993;2:2037–42. - PubMed
-
- Lee JH, Goto K, Matsuda C, et al. Characterization of a tandemly repeated 3.3-kb KpnI unit in the facioscapulohumeral muscular dystrophy (FSHD) gene region on chromosome 4q35. Muscle Nerve. 1995;2:S6–13. - PubMed
-
- Lunt PW, Jardine PE, Koch M, et al. Phenotypic-genotypic correlation will assist genetic counseling in 4q35-facioscapulohumeral muscular dystrophy. Muscle Nerve. 1995;2:S103–9. - PubMed
-
- Wijmenga C, Van Deutekom JC, Hewitt JE, et al. Pulsed-field gel electrophoresis of the D4F104S1 locus reveals the size and the parental origin of the facioscapulohumeral muscular dystrophy (FSHD)-associated deletions. Genomics. 1994;19:21–6. - PubMed
-
- Lunt PW, Jardine PE, Koch MC, et al. Correlation between fragment size at D4F104S1 and age at onset or at wheelchair use, with a possible generational effect, accounts for much phenotypic variation in 4q35-facioscapulohumeral muscular dystrophy (FSHD) Hum Mol Genet. 1995;4:951–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

