Leopard syndrome
- PMID: 18505544
- PMCID: PMC2467408
- DOI: 10.1186/1750-1172-3-13
Leopard syndrome
Abstract
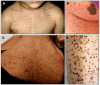
LEOPARD syndrome (LS, OMIM 151100) is a rare multiple congenital anomalies condition, mainly characterized by skin, facial and cardiac anomalies. LEOPARD is an acronym for the major features of this disorder, including multiple Lentigines, ECG conduction abnormalities, Ocular hypertelorism, Pulmonic stenosis, Abnormal genitalia, Retardation of growth, and sensorineural Deafness. About 200 patients have been reported worldwide but the real incidence of LS has not been assessed. Facial dysmorphism includes ocular hypertelorism, palpebral ptosis and low-set ears. Stature is usually below the 25th centile. Cardiac defects, in particular hypertrophic cardiomyopathy mostly involving the left ventricle, and ECG anomalies are common. The lentigines may be congenital, although more frequently manifest by the age of 4-5 years and increase throughout puberty. Additional common features are café-au-lait spots (CLS), chest anomalies, cryptorchidism, delayed puberty, hypotonia, mild developmental delay, sensorineural deafness and learning difficulties. In about 85% of the cases, a heterozygous missense mutation is detected in exons 7, 12 or 13 of the PTPN11 gene. Recently, missense mutations in the RAF1 gene have been found in two out of six PTPN11-negative LS patients. Mutation analysis can be carried out on blood, chorionic villi and amniotic fluid samples. LS is largely overlapping Noonan syndrome and, during childhood, Neurofibromatosis type 1-Noonan syndrome. Diagnostic clues of LS are multiple lentigines and CLS, hypertrophic cardiomyopathy and deafness. Mutation-based differential diagnosis in patients with borderline clinical manifestations is warranted. LS is an autosomal dominant condition, with full penetrance and variable expressivity. If one parent is affected, a 50% recurrence risk is appropriate. LS should be suspected in foetuses with severe cardiac hypertrophy and prenatal DNA test may be performed. Clinical management should address growth and motor development and congenital anomalies, in particular cardiac defects that should be monitored annually. Hypertrophic cardiomyopathy needs careful risk assessment and prophylaxis against sudden death in patients at risk. Hearing should be evaluated annually until adulthood. With the only exception of ventricular hypertrophy, adults with LS do not require special medical care and long-term prognosis is favourable.
Figures

References
-
- Gorlin RJ, Anderson RC, Blaw M. Multiple lentigines syndrome. Am J Dis Child. 1969;17:652–62. - PubMed
-
- Gorlin RJ, Anderson RC, Moller JH. The Leopard (multiple lentigines) syndrome revisited. Birth Defects Orig Artic Ser. 1971;7:110–5. - PubMed
-
- Zeisler EP, W S. Generalized Lentigo. Arch Dermat Syph. 1936;33:109–25.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

