Comparative genomic analysis of the gut bacterium Bifidobacterium longum reveals loci susceptible to deletion during pure culture growth
- PMID: 18505588
- PMCID: PMC2430713
- DOI: 10.1186/1471-2164-9-247
Comparative genomic analysis of the gut bacterium Bifidobacterium longum reveals loci susceptible to deletion during pure culture growth
Abstract
Background: Bifidobacteria are frequently proposed to be associated with good intestinal health primarily because of their overriding dominance in the feces of breast fed infants. However, clinical feeding studies with exogenous bifidobacteria show they don't remain in the intestine, suggesting they may lose competitive fitness when grown outside the gut.
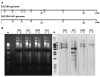
Results: To further the understanding of genetic attenuation that may be occurring in bifidobacteria cultures, we obtained the complete genome sequence of an intestinal isolate, Bifidobacterium longum DJO10A that was minimally cultured in the laboratory, and compared it to that of a culture collection strain, B. longum NCC2705. This comparison revealed colinear genomes that exhibited high sequence identity, except for the presence of 17 unique DNA regions in strain DJO10A and six in strain NCC2705. While the majority of these unique regions encoded proteins of diverse function, eight from the DJO10A genome and one from NCC2705, encoded gene clusters predicted to be involved in diverse traits pertinent to the human intestinal environment, specifically oligosaccharide and polyol utilization, arsenic resistance and lantibiotic production. Seven of these unique regions were suggested by a base deviation index analysis to have been precisely deleted from strain NCC2705 and this is substantiated by a DNA remnant from within one of the regions still remaining in the genome of NCC2705 at the same locus. This targeted loss of genomic regions was experimentally validated when growth of the intestinal B. longum in the laboratory for 1,000 generations resulted in two large deletions, one in a lantibiotic encoding region, analogous to a predicted deletion event for NCC2705. A simulated fecal growth study showed a significant reduced competitive ability of this deletion strain against Clostridium difficile and E. coli. The deleted region was between two IS30 elements which were experimentally demonstrated to be hyperactive within the genome. The other deleted region bordered a novel class of mobile elements, termed mobile integrase cassettes (MIC) substantiating the likely role of these elements in genome deletion events.
Conclusion: Deletion of genomic regions, often facilitated by mobile elements, allows bifidobacteria to adapt to fermentation environments in a very rapid manner (2 genome deletions per 1,000 generations) and the concomitant loss of possible competitive abilities in the gut.
Figures





References
-
- Yoshioka H, Iseki K, Fujita K. Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics. 1983;72:317–321. - PubMed
-
- Tissier H. Traitement des infections intestinales par la methode de la flore bacterienne de l'intestin. Crit Rev Soc Biol. 1906;60:359–361.
-
- Mitsuoka T, Hayakawa K. The fecal flora in man. I. Composition of the fecal flora of various age groups. Zentralbl Bakteriol [Orig A] 1973;223:333–342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

