Tissue transglutaminase modulates alpha-synuclein oligomerization
- PMID: 18505736
- PMCID: PMC2492824
- DOI: 10.1110/ps.036103.108
Tissue transglutaminase modulates alpha-synuclein oligomerization
Abstract
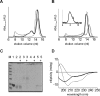
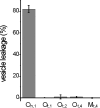
We have studied the interaction of the enzyme tissue transglutaminase (tTG), catalyzing cross-link formation between protein-bound glutamine residues and primary amines, with Parkinson's disease-associated alpha-synuclein protein variants at physiologically relevant concentrations. We have, for the first time, determined binding affinities of tTG for wild-type and mutant alpha-synucleins using surface plasmon resonance approaches, revealing high-affinity nanomolar equilibrium dissociation constants. Nanomolar tTG concentrations were sufficient for complete inhibition of fibrillization by effective alpha-synuclein cross-linking, resulting predominantly in intramolecularly cross-linked monomers accompanied by an oligomeric fraction. Since oligomeric species have a pathophysiological relevance we further investigated the properties of the tTG/alpha-synuclein oligomers. Atomic force microscopy revealed morphologically similar structures for oligomers from all alpha-synuclein variants; the extent of oligomer formation was found to correlate with tTG concentration. Unlike normal alpha-synuclein oligomers the resultant structures were extremely stable and resistant to GdnHCl and SDS. In contrast to normal beta-sheet-containing oligomers, the tTG/alpha-synuclein oligomers appear to be unstructured and are unable to disrupt phospholipid vesicles. These data suggest that tTG binds equally effective to wild-type and disease mutant alpha-synuclein variants. We propose that tTG cross-linking imposes structural constraints on alpha-synuclein, preventing the assembly of structured oligomers required for disruption of membranes and for progression into fibrils. In general, cross-linking of amyloid forming proteins by tTG may prevent the progression into pathogenic species.
Figures


Similar articles
-
Presence of tissue transglutaminase in granular endoplasmic reticulum is characteristic of melanized neurons in Parkinson's disease brain.Brain Pathol. 2011 Mar;21(2):130-9. doi: 10.1111/j.1750-3639.2010.00429.x. Epub 2010 Aug 20. Brain Pathol. 2011. PMID: 20731657 Free PMC article.
-
Dissecting the mechanisms of tissue transglutaminase-induced cross-linking of alpha-synuclein: implications for the pathogenesis of Parkinson disease.J Biol Chem. 2009 May 8;284(19):13128-42. doi: 10.1074/jbc.M809067200. Epub 2009 Jan 21. J Biol Chem. 2009. PMID: 19164286 Free PMC article.
-
Blockade of enzyme activity inhibits tissue transglutaminase-mediated transamidation of α-synuclein in a cellular model of Parkinson's disease.Neurochem Int. 2011 Jun;58(7):785-93. doi: 10.1016/j.neuint.2011.03.004. Epub 2011 Mar 31. Neurochem Int. 2011. PMID: 21440023
-
Navigating α-Synuclein Aggregation Inhibition: Methods, Mechanisms, and Molecular Targets.Chem Rec. 2024 Feb;24(2):e202300282. doi: 10.1002/tcr.202300282. Epub 2023 Nov 2. Chem Rec. 2024. PMID: 37919046 Review.
-
α-Synuclein oligomers: an amyloid pore? Insights into mechanisms of α-synuclein oligomer-lipid interactions.Mol Neurobiol. 2013 Apr;47(2):613-21. doi: 10.1007/s12035-012-8331-4. Epub 2012 Sep 6. Mol Neurobiol. 2013. PMID: 22956232 Review.
Cited by
-
Absence of tissue transglutaminase reduces amyloid-beta pathology in APP23 mice.Neuropathol Appl Neurobiol. 2022 Jun;48(4):e12796. doi: 10.1111/nan.12796. Epub 2022 Feb 23. Neuropathol Appl Neurobiol. 2022. PMID: 35141929 Free PMC article.
-
Metabolic Investigations of the Molecular Mechanisms Associated with Parkinson's Disease.Metabolites. 2017 May 24;7(2):22. doi: 10.3390/metabo7020022. Metabolites. 2017. PMID: 28538683 Free PMC article. Review.
-
Human tissue transglutaminase is inhibited by pharmacologic and chemical acetylation.Protein Sci. 2010 Feb;19(2):229-35. doi: 10.1002/pro.301. Protein Sci. 2010. PMID: 19998405 Free PMC article.
-
Presence of tissue transglutaminase in granular endoplasmic reticulum is characteristic of melanized neurons in Parkinson's disease brain.Brain Pathol. 2011 Mar;21(2):130-9. doi: 10.1111/j.1750-3639.2010.00429.x. Epub 2010 Aug 20. Brain Pathol. 2011. PMID: 20731657 Free PMC article.
-
The Role of α-Synuclein and LRRK2 in Tau Phosphorylation.Parkinsons Dis. 2015;2015:734746. doi: 10.1155/2015/734746. Epub 2015 Apr 21. Parkinsons Dis. 2015. PMID: 25977830 Free PMC article. Review.
References
-
- Andringa, G., Lam, K.Y., Chegary, M., Wang, X., Chase, T.N., Bennett, M.C. Tissue transglutaminase catalyzes the formation of α-synuclein crosslinks in Parkinson's disease. FASEB J. 2004;18:932–934. - PubMed
-
- Bendit, E.G., Ross, D. A technique for obtaining the ultraviolet absorption spectrum of solid keratin. Appl. Spectros. 1961;15:103–105.
-
- Chen, P.S., Toribara, T.Y., Warner, H. Microdetermination of phosphorus. Anal. Chem. 1956;28:1756–1758.
-
- Eliezer, D., Kutluay, E., Bussell R., Jr, Browne, G. Conformational properties of α-synuclein in its free and lipid-associated states. J. Mol. Biol. 2001;307:1061–1073. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

