A comprehensive in vitro and in silico analysis of antibiotics that activate pregnane X receptor and induce CYP3A4 in liver and intestine
- PMID: 18505790
- PMCID: PMC4664062
- DOI: 10.1124/dmd.108.020701
A comprehensive in vitro and in silico analysis of antibiotics that activate pregnane X receptor and induce CYP3A4 in liver and intestine
Abstract
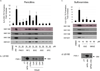
We have investigated several in silico and in vitro methods to improve our ability to predict potential drug interactions of antibiotics. Our focus was to identify those antibiotics that activate pregnane X receptor (PXR) and induce CYP3A4 in human hepatocytes and intestinal cells. Human PXR activation was screened using reporter assays in HepG2 cells, kinetic measurements of PXR activation were made in DPX-2 cells, and induction of CYP3A4 expression and activity was verified by quantitative polymerase chain reaction, immunoblotting, and testosterone 6beta-hydroxylation in primary human hepatocytes and LS180 cells. We found that in HepG2 cells CYP3A4 transcription was activated strongly (> 10-fold) by rifampin and troleandomycin; moderately (> or = 7-fold) by dicloxacillin, tetracycline, clindamycin, griseofulvin, and (> or = 4-fold) erythromycin; and weakly (> 2.4-fold) by nafcillin, cefaclor, sulfisoxazole, and (> 2-fold) cefadroxil and penicillin V. Similar although not identical results were obtained in DPX-2 cells. CYP3A4 mRNA and protein expression were induced by these antibiotics to differing extents in both liver and intestinal cells. CYP3A4 activity was significantly increased by rifampin (9.7-fold), nafcillin and dicloxacillin (5.9-fold), and weakly induced (2-fold) by tetracycline, sufisoxazole, troleandomycin, and clindamycin. Multiple pharmacophore models and docking indicated a good fit for dicloxacillin and nafcillin in PXR. These results suggest that in vitro and in silico methods can help to prioritize and identify antibiotics that are most likely to reduce exposures of medications (such as oral contraceptive agents) which interact with enzymes and transporters regulated by PXR. In summary, nafcillin, dicloxacillin, cephradine, tetracycline, sulfixoxazole, erythromycin, clindamycin, and griseofulvin exhibit a clear propensity to induce CYP3A4 and warrant further clinical investigation.
Figures



References
-
- Atkinson A, Kenny JR, Grime K. Automated assessment of time-dependent inhibition of human cytochrome P450 enzymes using liquid chromatography-tandem mass spectrometry analysis. Drug Metab Dispos. 2005;33:1637–1647. - PubMed
-
- Bachmann K, Patel H, Batayneh Z, Slama J, White D, Posey J, Ekins S, Gold D, Sambucetti L. PXR and the regulation of apoA1 and HDL-cholesterol in rodents. Pharmacol Res. 2004;50:237–246. - PubMed
-
- Back DJ, Tjia J, Martin C, Millar E, Mant T, Morrison P, Orme M. The lack of interaction between temafloxacin and combined oral contraceptive steroids. Contraception. 1991;43:317–323. - PubMed
-
- Barditch-Crovo P, Trapnell CB, Ette E, Zacur HA, Coresh J, Rocco LE, Hendrix CW, Flexner C. The effects of rifampin and rifabutin on the pharmacokinetics and pharmacodynamics of a combination oral contraceptive. Clin Pharmacol Ther. 1999;65:428–438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

