Hookworm-induced persistent changes to the immunological environment of the lung
- PMID: 18505812
- PMCID: PMC2493237
- DOI: 10.1128/IAI.00192-08
Hookworm-induced persistent changes to the immunological environment of the lung
Abstract
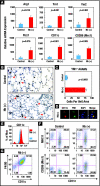
A number of important helminth parasites of humans have incorporated short-term residence in the lungs as an obligate phase of their life cycles. The significance of this transient pulmonary exposure to the infection and immunity is not clear. Employing a rodent model of infection with hookworm (Nippostrongylus brasiliensis), we characterized the long-term changes in the immunological status of the lungs induced by parasite infection. At 36 days after infection, alterations included a sustained increase in the transcription of both Th2 and Th1 cytokines as well as a significant increase in the number and frequency of alveolar macrophages displaying an alternatively activated phenotype. While N. brasiliensis did not induce alternate activation of lung macrophages in STAT6(-/-) animals, the parasite did induce a robust Th17 response in the pulmonary environment, suggesting that STAT6 signaling plays a role in modulating Th17 immunity and pathology in the lungs. In the context of the cellular and molecular changes induced by N. brasiliensis infection, there was a significant reduction in overall airway responsiveness and lung inflammation in response to allergen. In addition, the N. brasiliensis-altered pulmonary environment showed dramatic alterations in the nature and number of genes that were up- and downregulated in the lung in response to allergen challenge. The results demonstrate that even a transient exposure to a helminth parasite can effect significant and protracted changes in the immunological environment of the lung and that these complex molecular and cellular changes are likely to play a role in modulating a subsequent allergen-induced inflammatory response.
Figures







References
-
- Allen, J. E., and R. M. Maizels. 1996. Immunology of human helminth infection. Int. Arch. Allergy Immunol. 1093-10. - PubMed
-
- Araujo, M. I., A. A. Lopes, M. Medeiros, A. A. Cruz, L. Sousa-Atta, D. Sole, and E. M. Carvalho. 2000. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int. Arch. Allergy Immunol. 123145-148. - PubMed
-
- Arora, S., Y. Hernandez, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 1746346-6356. - PubMed
-
- Bashir, M. E., P. Andersen, I. J. Fuss, H. N. Shi, and C. Nagler-Anderson. 2002. An enteric helminth infection protects against an allergic response to dietary antigen. J. Immunol. 1693284-3292. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

