Mind bomb-1 is essential for intraembryonic hematopoiesis in the aortic endothelium and the subaortic patches
- PMID: 18505817
- PMCID: PMC2493361
- DOI: 10.1128/MCB.00436-08
Mind bomb-1 is essential for intraembryonic hematopoiesis in the aortic endothelium and the subaortic patches
Abstract
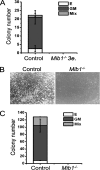
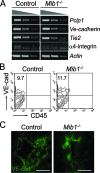
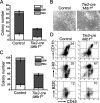
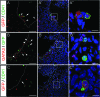
Intraembryonic hematopoiesis occurs at two different sites, the floor of the aorta and subaortic patches (SAPs) of the para-aortic splanchnopleura (P-Sp)/aorta-gonad-mesonephros (AGM) region. Notch1 and RBP-jkappa are critical for the specification of hematopoietic stem cells (HSCs) in Notch signal-receiving cells. However, the mechanism by which Notch signaling is triggered from the Notch signal-sending cells to support embryonic hematopoiesis remains to be determined. We previously reported that Mind bomb-1 (Mib1) regulates Notch ligands in the Notch signal-sending cells (B. K. Koo, M. J. Yoon, K. J. Yoon, S. K. Im, Y. Y. Kim, C. H. Kim, P. G. Suh, Y. N. Jan, and Y. Y. Kong, PLoS ONE 2:e1221, 2007). Here, we show that intraembryonic hematopoietic progenitors were absent in the P-Sp of Mib1(-/-) embryos, whereas they were partly preserved in the Tie2-cre; Mib1(f)(/f) P-Sps, suggesting that Mib1 plays a role in the endothelium and the SAPs. Interestingly, dll1 and dll4/Jag1 are expressed in the SAPs and the endothelium of the AGM, respectively, where mib1 is detected. Indeed, Notch signaling was activated in the nascent HSCs at both sites. In the P-Sp explant culture, the overexpression of Dll1 in OP9 stromal cells rescued the failed production of hematopoietic progenitors in the Mib1(-/-) P-Sp, while its activity was abolished by Mib1 knockdown. These results suggest that Mib1 is important for intraembryonic hematopoiesis not only in the aortic endothelium but also in the SAPs.
Figures



References
-
- Artavanis-Tsakonas, S., M. D. Rand, and R. J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science 284770-776. - PubMed
-
- Brou, C., F. Logeat, N. Gupta, C. Bessia, O. LeBail, J. R. Doedens, A. Cumano, P. Roux, R. A. Black, and A. Israel. 2000. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell 5207-216. - PubMed
-
- Corbel, C., and J. Salaun. 2002. AlphaIIb integrin expression during development of the murine hemopoietic system. Dev. Biol. 243301-311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
