Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas
- PMID: 18505913
- PMCID: PMC3886801
- DOI: 10.1158/1541-7786.MCR-07-2180
Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas
Abstract
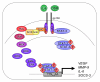
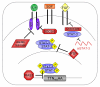
Glioblastoma is the most common and severe primary brain tumor in adults. Its aggressive and infiltrative nature renders the current therapeutics of surgical resection, radiation, and chemotherapy relatively ineffective. Accordingly, recent research has focused on the elucidation of various signal transduction pathways in glioblastoma, particularly aberrant activation. This review focuses on the signal transducer and activator of transcription-3 (STAT-3) signal transduction pathway in the context of this devastating tumor. STAT-3 is aberrantly activated in human glioblastoma tissues, and this activation is implicated in controlling critical cellular events thought to be involved in gliomagenesis, such as cell cycle progression, apoptosis, angiogenesis, and immune evasion. There are no reports of gain-of-function mutations in glioblastoma; rather, the activation of STAT-3 is thought to be a consequence of either dysregulation of upstream kinases or loss of endogenous inhibitors. This review provides detailed insight into the multiple mechanisms of STAT-3 activation in glioblastoma, as well as describing endogenous and chemical inhibitors of this pathway and their clinical significance. In glioblastoma, STAT-3 acts a molecular hub to link extracellular signals to transcriptional control of proliferation, cell cycle progression, and immune evasion. Because STAT-3 plays this central role in glioblastoma signal transduction, it has significant potential as a therapeutic target.
Figures


References
-
- Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol (Berl) 2005;109:93–108. - PubMed
-
- Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494–503. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Rao RD, James CD. Altered molecular pathways in gliomas: an overview of clinically relevant issues. Semin Oncol. 2004;31:595–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

