Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial
- PMID: 18505948
- PMCID: PMC2743160
- DOI: 10.1001/jama.299.20.2391
Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial
Erratum in
- JAMA. 2009 Mar 11;301(10):1024
Abstract
Context: Depression occurs in more than half of patients who have experienced a stroke. Poststroke depression has been shown in numerous studies to be associated with both impaired recovery in activities of daily living and increased mortality. Prevention of depression thus represents a potentially important goal.
Objective: To determine whether treatment with escitalopram or problem-solving therapy over the first year following acute stroke will decrease the number of depression cases that develop compared with placebo medication.
Design, setting, and participants: A multisite randomized controlled trial for prevention of depression among 176 nondepressed patients was conducted within 3 months following acute stroke from July 9, 2003, to October 1, 2007. The 12-month trial included 3 groups: a double-blind placebo-controlled comparison of escitalopram (n = 59) with placebo (n = 58), and a nonblinded problem-solving therapy group (n = 59).
Main outcome measures: The main outcome measure was the development of major or minor poststroke depression based on symptoms elicited by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) and the diagnostic criteria from DSM-IV for depression due to stroke with major depressive-like episode or minor depression (ie, research criteria).
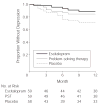
Results: Patients who received placebo were significantly more likely to develop depression than individuals who received escitalopram (11 major and 2 minor cases of depression [22.4%] vs 3 major and 2 minor cases of depression [8.5%], adjusted hazard ratio [HR], 4.5; 95% confidence interval [CI], 2.4-8.2; P < .001) and also more likely than individuals who received problem-solving therapy (5 major and 2 minor cases of depression [11.9%], adjusted HR, 2.2; 95% CI, 1.4-3.5; P < .001). These results were adjusted for history of mood disorders and remained significant after considering possible confounders such as age, sex, treatment site, and severity of impairment in the model. Using an intention-to-treat conservative method of analyzing the data, which assumed that all 27 patients who did not start randomized treatment would have developed depression, and controlling for prior history of mood disorders, escitalopram was superior to placebo (23.1% vs 34.5%; adjusted HR, 2.2; 95% CI, 1.2-3.9; P = .007), while problem-solving therapy was not significantly better than placebo (30.5% vs 34.5%; adjusted HR, 1.1; 95% CI, 0.8-1.5; P = .51). Adverse events, including all-cause hospitalizations, nausea, and adverse effects associated with escitalopram were not significantly different between the 3 groups.
Conclusions: In this study of nondepressed patients with recent stroke, the use of escitalopram or problem-solving therapy resulted in a significantly lower incidence of depression over 12 months of treatment compared with placebo, but problem-solving therapy did not achieve significant results over placebo using the intention-to-treat conservative method of analysis.
Trial registration: clinicaltrials.gov Identifier: NCT00071643.
Figures

Comment in
-
Escitalopram, problem-solving therapy, and poststroke depression.JAMA. 2008 Oct 15;300(15):1757-8; author reply 1758-9. doi: 10.1001/jama.300.15.1757-c. JAMA. 2008. PMID: 18854528 No abstract available.
-
Escitalopram, problem-solving therapy, and poststroke depression.JAMA. 2008 Oct 15;300(15):1757; author reply 1758-9. doi: 10.1001/jama.300.15.1757-b. JAMA. 2008. PMID: 18854529 No abstract available.
-
Escitalopram, problem-solving therapy, and poststroke depression.JAMA. 2008 Oct 15;300(15):1757; author reply 1758-9. doi: 10.1001/jama.300.15.1757-a. JAMA. 2008. PMID: 18854530 No abstract available.
-
Escitalopram, problem-solving therapy, and poststroke depression.JAMA. 2008 Oct 15;300(15):1758; author reply 1758-9. doi: 10.1001/jama.300.15.1758-a. JAMA. 2008. PMID: 18854531 No abstract available.
-
Escitalopram and problem solving therapy reduce the incidence of post-stroke depression.Evid Based Ment Health. 2009 Feb;12(1):10. doi: 10.1136/ebmh.12.1.10. Evid Based Ment Health. 2009. PMID: 19176769 No abstract available.
-
Incomplete financial disclosure in a study of escitalopram and problem-solving therapy for prevention of poststroke depression.JAMA. 2009 Mar 11;301(10):1023-4. doi: 10.1001/jama.2009.256. JAMA. 2009. PMID: 19278945 No abstract available.
References
-
- Caplan GA. A Conceptual Model for Primary Prevention: Principles of Preventive Psychiatry. New York NY: Basic Books; 1964.
-
- Mrazek PJ, Haggerty RJ, editors. Committee on Prevention of Mental Disorders; Division of Biobehavioral Sciences and Mental Disorders. Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research. Washington, DC: National Academy Press; 1994. - PubMed
-
- Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–e151. - PubMed
-
- Aström M, Adolfsson R, Asplund K. Major depression in stroke patients: a 3-year longitudinal study. Stroke. 1993;24(7):976–982. - PubMed
-
- Berg A, Psych L, Palomaki H, et al. Poststroke depression—an 18-month follow-up. Stroke. 2003;34(1):138–143. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

