Oxaliplatin-DNA adduct formation in white blood cells of cancer patients
- PMID: 18506148
- PMCID: PMC2441951
- DOI: 10.1038/sj.bjc.6604387
Oxaliplatin-DNA adduct formation in white blood cells of cancer patients
Abstract
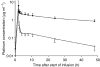
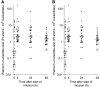
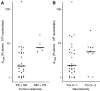
In this study, we investigated the kinetics of oxaliplatin-DNA adduct formation in white blood cells of cancer patients in relation to efficacy as well as oxaliplatin-associated neurotoxicity. Thirty-seven patients with various solid tumours received 130 mg m(-2) oxaliplatin as a 2-h infusion. Oxaliplatin-DNA adduct levels were measured in the first cycle using adsorptive stripping voltammetry. Platinum concentrations were measured in ultrafiltrate and plasma using a validated flameless atomic absorption spectrometry method. DNA adduct levels showed a characteristic time course, but were not correlated to platinum pharmacokinetics and varied considerably among individuals. In patients showing tumour response, adduct levels after 24 and 48 h were significantly higher than in nonresponders. Oxaliplatin-induced neurotoxicity was more pronounced but was not significantly different in patients with high adduct levels. The potential of oxaliplatin-DNA adduct measurements as pharmacodynamic end point should be further investigated in future trials.
Figures


Similar articles
-
Administration of reduced glutathione in FOLFOX4 adjuvant treatment for colorectal cancer: effect on oxaliplatin pharmacokinetics, Pt-DNA adduct formation, and neurotoxicity.Anticancer Drugs. 2009 Jun;20(5):396-402. doi: 10.1097/CAD.0b013e32832a2dc1. Anticancer Drugs. 2009. PMID: 19287306 Clinical Trial.
-
Pharmacokinetics of oxaliplatin (NSC 266046) alone and in combination with paclitaxel in cancer patients.Cancer Chemother Pharmacol. 2002 May;49(5):367-74. doi: 10.1007/s00280-002-0426-6. Epub 2002 Feb 20. Cancer Chemother Pharmacol. 2002. PMID: 11976830 Clinical Trial.
-
Platinum-DNA adduct formation in leucocytes of children in relation to pharmacokinetics after cisplatin and carboplatin therapy.Br J Cancer. 1997;76(11):1466-73. doi: 10.1038/bjc.1997.579. Br J Cancer. 1997. PMID: 9400943 Free PMC article.
-
Cellular and molecular pharmacology of oxaliplatin.Mol Cancer Ther. 2002 Jan;1(3):227-35. Mol Cancer Ther. 2002. PMID: 12467217 Review.
-
Oxaliplatin: pharmacokinetics and chronopharmacological aspects.Clin Pharmacokinet. 2000 Jan;38(1):1-21. doi: 10.2165/00003088-200038010-00001. Clin Pharmacokinet. 2000. PMID: 10668856 Review.
Cited by
-
Time course of DNA adduct formation in peripheral blood granulocytes and lymphocytes after drinking alcohol.Mutagenesis. 2012 Jul;27(4):485-90. doi: 10.1093/mutage/ges008. Epub 2012 Mar 9. Mutagenesis. 2012. PMID: 22406526 Free PMC article.
-
Development and Application of Physiologically-Based Pharmacokinetic Model to Predict Systemic and Organ Exposure of Colorectal Cancer Drugs.Pharmaceutics. 2025 Jan 3;17(1):57. doi: 10.3390/pharmaceutics17010057. Pharmaceutics. 2025. PMID: 39861705 Free PMC article.
-
Review of Cisplatin and oxaliplatin in current immunogenic and monoclonal antibody treatments.Oncol Rev. 2014 Sep 23;8(2):256. doi: 10.4081/oncol.2014.256. eCollection 2014 Sep 23. Oncol Rev. 2014. PMID: 25992242 Free PMC article. Review.
-
Microdose-Induced Drug-DNA Adducts as Biomarkers of Chemotherapy Resistance in Humans and Mice.Mol Cancer Ther. 2017 Feb;16(2):376-387. doi: 10.1158/1535-7163.MCT-16-0381. Epub 2016 Nov 30. Mol Cancer Ther. 2017. PMID: 27903751 Free PMC article.
-
Oxaliplatin: a review in the era of molecularly targeted therapy.Curr Oncol. 2011 Jan;18(1):18-25. doi: 10.3747/co.v18i1.708. Curr Oncol. 2011. PMID: 21331278 Free PMC article.
References
-
- Allain P, Brienza S, Gamelin R, Taamma A, Krikorian A, Turcant C, Cvitkovic E, Misset JL (1996) Oxaliplatin induced platinum DNA adducts in white blood cells of cancer patients. Proc Annu Meet Am Assoc Cancer Res 37: 404
-
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. New Engl J Med 350: 2343–2351 - PubMed
-
- Boffetta P, Fichtinger-Schepman AM, Weiderpass E, van Dijk-Knijnenburg HC, Stoter G, van Oosterom AT, Keizer HJ, Fosså SD, Kaldor J, Roy P (1998) Cisplatin-DNA adducts and proteinbound platinum in blood of testicular cancer patients. Anticancer Drugs 9: 125–129 - PubMed
-
- Bonetti A, Apostoli P, Zaninelli M, Pavanel F, Colombatti M, Cetto GL, Franceschi T, Sperotto L, Leone R (1996) Inductively coupled plasma mass spectroscopy quantitation of platinum-DNA adducts in peripheral blood leukocytes of patients receiving cisplatin- or carboplatin-based chemotherapy. Clin Cancer Res 2: 1829–1835 - PubMed
-
- Cassidy J, Misset JL (2002) Oxaliplatin-related side effects: characteristics and management. Semin Oncol 29(suppl 15): 11–20 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

