Cisplatin alters nitric oxide synthase levels in human ovarian cancer cells: involvement in p53 regulation and cisplatin resistance
- PMID: 18506185
- PMCID: PMC2410127
- DOI: 10.1038/sj.bjc.6604375
Cisplatin alters nitric oxide synthase levels in human ovarian cancer cells: involvement in p53 regulation and cisplatin resistance
Abstract
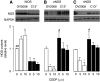
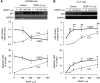
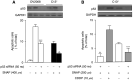
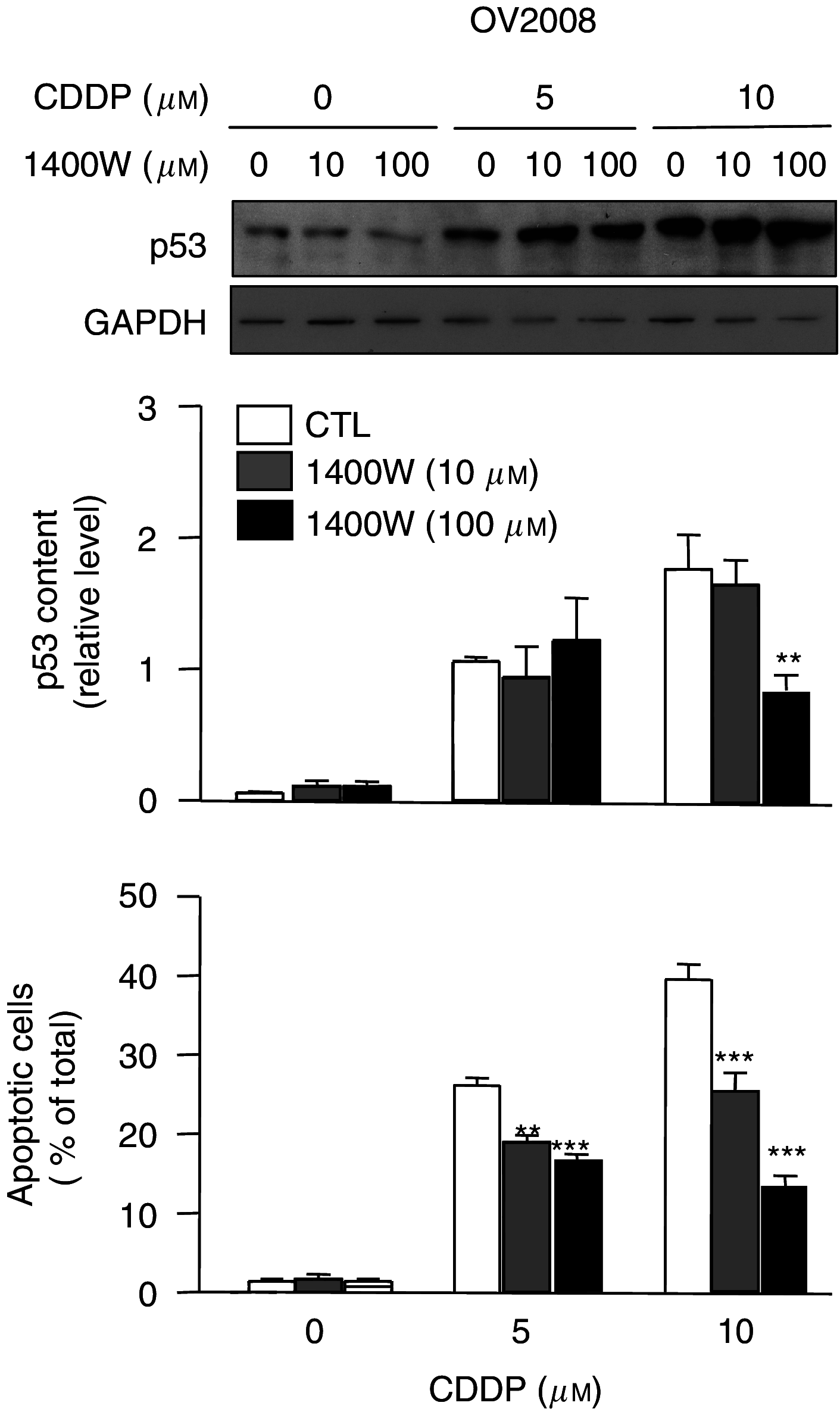
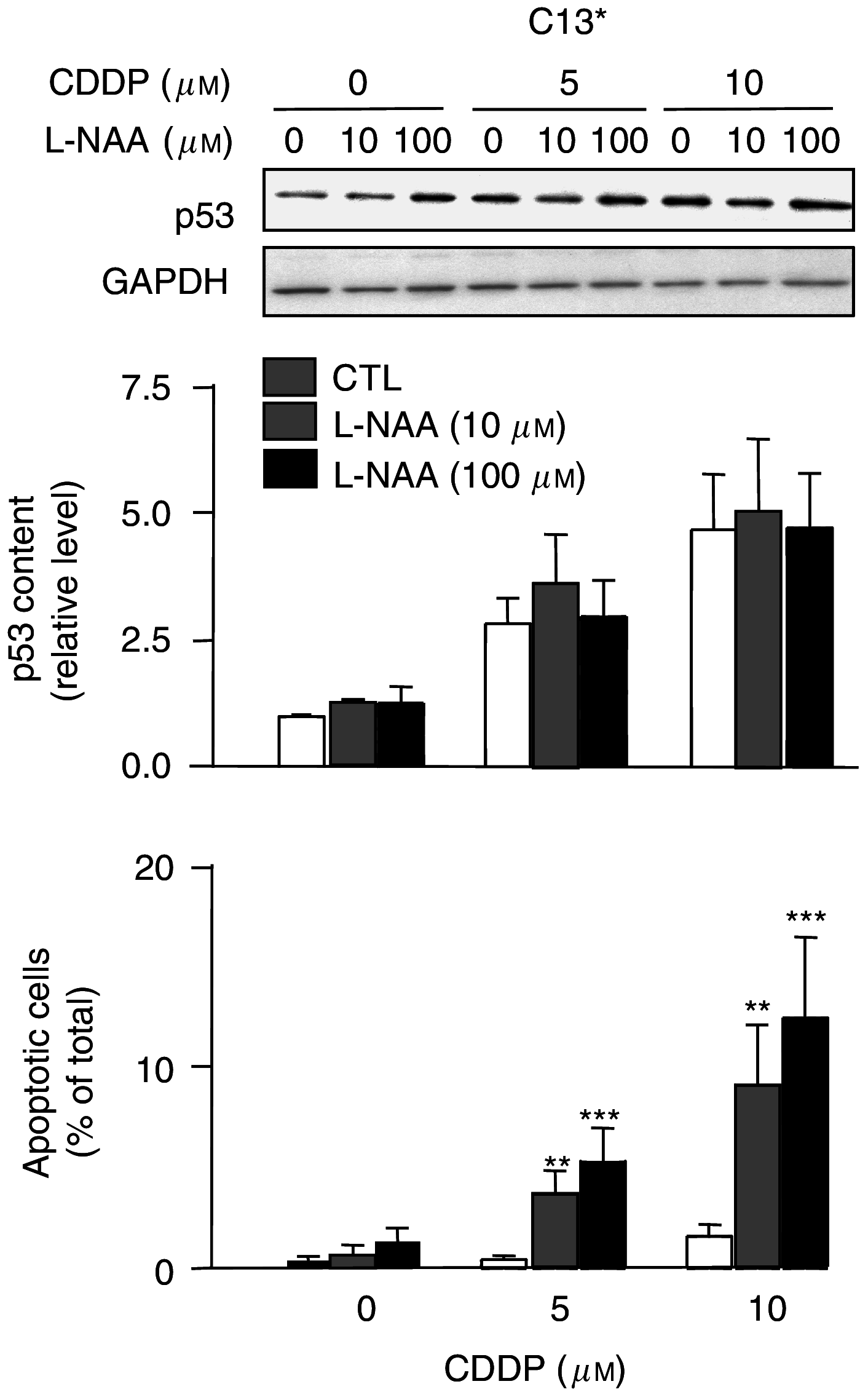
The present study determines if (1) basal protein levels of nitric oxide (NO) synthases (eNOS, iNOS, and nNOS) are different in cisplatin-sensitive (OV2008) and counterpart cisplatin-resistant (C13(*)) human ovarian cancer cells, (2) cisplatin alters NOS levels, (3) NO donor causes apoptosis and p53 upregulation, (4) NO donor sensitizes C13(*) cells to cisplatin via p53 upregulation (determined by p53 siRNA gene-knockdown), and (5) inhibition of endogenous NOS alters cisplatin-induced apoptosis. Basal iNOS levels were higher in OV2008 cells than in C13(*) cells. Cisplatin upregulated iNOS, but dramatically reduced eNOS and nNOS, in OV2008 cells only. Failure of cisplatin to upregulate iNOS and downregulate eNOS/nNOS in cisplatin-resistant C13(*) cells may be an aetiological factor in the development of resistance. The NO donor S-nitroso-N-acetylpenicillamine (SNAP) increased p53 protein levels and induced apoptosis in both cell types, and enhanced cisplatin-induced apoptosis in C13(*) cells in a p53-dependent manner (i.e., enhancement blocked by p53 siRNA). Specific iNOS inhibitor 1400W partially blocked cisplatin-induced apoptosis in OV2008 cells. In cisplatin-resistant C13(*) cells, blocking all NOSs with N(G)-amino-L-arginine dramatically changed these cells from cisplatin-resistant to cisplatin-sensitive, greatly potentiating cisplatin-induced apoptosis. The data suggest important roles for the three NOSs in regulating chemoresistance to cisplatin in ovarian cancer cells.
Figures
References
-
- Ambs S, Hussain SP, Harris CC (1997) Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J 11: 443–448 - PubMed
-
- Bellamy TC, Griffiths C, Garthwaite J (2002) Differential sensitivity of guanylyl cyclase and mitochondrial respiration to nitric oxide measured using clamped concentrations. J Biol Chem 277: 31801–31807 - PubMed
-
- Chan SL, Fiscus RR (2003) Guanylyl cyclase inhibitors NS2028 and ODQ and protein kinase G (PKG) inhibitor KT5823 trigger apoptotic DNA fragmentation in immortalized uterine epithelial cells: anti-apoptotic effects of basal cGMP/PKG. Mol Hum Reprod 9: 775–783 - PubMed
-
- Cheng Chew SB, Leung PY, Fiscus RR (2003) Preincubation with atrial natriuretic peptide protects NG108-15 cells against the toxic/proapoptotic effects of the nitric oxide donor S-nitroso-N-acetylpenicillamine. Histochem Cell Biol 120: 163–171 - PubMed
-
- Fiscus RR (2002) Involvement of cyclic GMP and protein kinase G in the regulation of apoptosis and survival in neural cells. Neurosignals 11: 175–190 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

