Functional genomics and SNP analysis of human genes encoding proline metabolic enzymes
- PMID: 18506409
- PMCID: PMC2707926
- DOI: 10.1007/s00726-008-0107-9
Functional genomics and SNP analysis of human genes encoding proline metabolic enzymes
Abstract
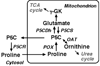
Proline metabolism in mammals involves two other amino acids, glutamate and ornithine, and five enzymatic activities, Delta(1)-pyrroline-5-carboxylate (P5C) reductase (P5CR), proline oxidase, P5C dehydrogenase, P5C synthase and ornithine-delta-aminotransferase (OAT). With the exception of OAT, which catalyzes a reversible reaction, the other four enzymes are unidirectional, suggesting that proline metabolism is purpose-driven, tightly regulated, and compartmentalized. In addition, this tri-amino-acid system also links with three other pivotal metabolic systems, namely the TCA cycle, urea cycle, and pentose phosphate pathway. Abnormalities in proline metabolism are relevant in several diseases: six monogenic inborn errors involving metabolism and/or transport of proline and its immediate metabolites have been described. Recent advances in the Human Genome Project, in silico database mining techniques, and research in dissecting the molecular basis of proline metabolism prompted us to utilize functional genomic approaches to analyze human genes which encode proline metabolic enzymes in the context of gene structure, regulation of gene expression, mRNA variants, protein isoforms, and single nucleotide polymorphisms.
Figures

References
-
- Baumgartner MR, Hu CAA, Almashanu S, Steel G, Obie C, Aral B, Rabier D, Kamoun P, Saudubray J-M, Valle D. Hyperammonemia with reduced ornithine, citrulline, arginine and proline: a new inborn error caused by a mutation in the gene encoding delta-1-pyrroline-5-carboxylate synthase. Hum Mol Gen. 2000;9:2853–2858. - PubMed
-
- Böck A, Forchhammer K, Heider J, Leinfelder W, Sawers G, Veprek B, Zinoni F. Selenocysteine: the 21st amino acid. Mol. Microbiol. 1991;5:515–520. - PubMed
-
- Brody LC, Mitchell GA, Obie C, Michaud J, Steel G, Fontaine G, Robert MF, Sipila I, Kaiser-Kupfer M, Valle D. Ornithine delta aminotransferase mutations in gyrate atrophy. Allelic heterogeneity and functional consequences. J Biol Chem. 1992;267:3302–3307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

