AxCaliber: a method for measuring axon diameter distribution from diffusion MRI
- PMID: 18506799
- PMCID: PMC4667732
- DOI: 10.1002/mrm.21577
AxCaliber: a method for measuring axon diameter distribution from diffusion MRI
Abstract
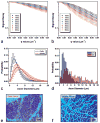
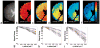
The diameter of a myelinated nerve axon is directly proportional to its conduction velocity, so the axon diameter distribution helps determine the channel capacity of nervous transmission along fascicles in the central (CNS) and peripheral nervous systems (PNS). Previously, this histological information could only be obtained using invasive tissue biopsies. Here we propose a new NMR-based approach that employs a model of water diffusion within "restricted" cylindrical axons to estimate their diameter distribution within a nerve bundle. This approach can be combined with MRI to furnish an estimate of the axon diameter distribution within each voxel. This method is validated by comparing the diameter distributions measured using the NMR and histological techniques on sciatic and optic nerve tissue specimens. The axon diameter distribution measured in each voxel of porcine spinal cord using MRI and using histological methods were similar. Applications are expected in longitudinal studies designed to follow nerve growth in normal and abnormal development, as well as in diagnosing disorders and diseases affecting specific populations of axons in the CNS and PNS.
Copyright (c) 2008 Wiley-Liss, Inc.
Figures


References
-
- Hursh JB. The properties of growing nerve fibers. Am J Physiol. 1939;127:140–153.
-
- Ritchie JM. On the relation between fibre diameter and conduction velocity in myelinated nerve fibres. Proc R Soc Lond B Biol Sci. 1982;217:29–35. - PubMed
-
- Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3:141–150. - PubMed
-
- Tasaki I, Ishii K, Ito H. On the relation between the conduction-rate, the fiber-diameter and the internodal distance of the medullated nerve fiber. Jpn J Med Sci III Biophys. 1943;9:189–199.
-
- Nicholls JG, Martin AR, Wallace BG, Fuchs PA. From neuron to brain. Sunderland, MA: Sinauer Associates; 2001.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

