Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity
- PMID: 18507503
- PMCID: PMC2430908
- DOI: 10.1371/journal.pbio.0060123
Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity
Abstract
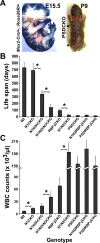
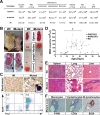
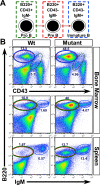
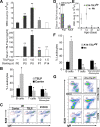
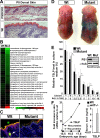
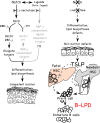
Epidermal keratinocytes form a highly organized stratified epithelium and sustain a competent barrier function together with dermal and hematopoietic cells. The Notch signaling pathway is a critical regulator of epidermal integrity. Here, we show that keratinocyte-specific deletion of total Notch signaling triggered a severe systemic B-lymphoproliferative disorder, causing death. RBP-j is the DNA binding partner of Notch, but both RBP-j-dependent and independent Notch signaling were necessary for proper epidermal differentiation and lipid deposition. Loss of both pathways caused a persistent defect in skin differentiation/barrier formation. In response, high levels of thymic stromal lymphopoietin (TSLP) were released into systemic circulation by Notch-deficient keratinocytes that failed to differentiate, starting in utero. Exposure to high TSLP levels during neonatal hematopoiesis resulted in drastic expansion of peripheral pre- and immature B-lymphocytes, causing B-lymphoproliferative disorder associated with major organ infiltration and subsequent death, a previously unappreciated systemic effect of TSLP. These observations demonstrate that local skin perturbations can drive a lethal systemic disease and have important implications for a wide range of humoral and autoimmune diseases with skin manifestations.
Conflict of interest statement
Figures


Comment in
-
When skin damage causes death.PLoS Biol. 2008 May;6(5):e133. doi: 10.1371/journal.pbio.0060133. Epub 2008 May 27. PLoS Biol. 2008. PMID: 20076711 Free PMC article. No abstract available.
References
-
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, et al. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. - PubMed
-
- Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–E108. - PubMed
-
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

