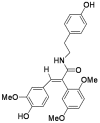
Squamosamide derivative FLZ protects dopaminergic neurons against inflammation-mediated neurodegeneration through the inhibition of NADPH oxidase activity
- PMID: 18507839
- PMCID: PMC2413210
- DOI: 10.1186/1742-2094-5-21
Squamosamide derivative FLZ protects dopaminergic neurons against inflammation-mediated neurodegeneration through the inhibition of NADPH oxidase activity
Abstract
Background: Inflammation plays an important role in the pathogenesis of Parkinson's disease (PD) through over-activation of microglia, which consequently causes the excessive production of proinflammatory and neurotoxic factors, and impacts surrounding neurons and eventually induces neurodegeneration. Hence, prevention of microglial over-activation has been shown to be a prime target for the development of therapeutic agents for inflammation-mediated neurodegenerative diseases.
Methods: For in vitro studies, mesencephalic neuron-glia cultures and reconstituted cultures were used to investigate the molecular mechanism by which FLZ, a squamosamide derivative, mediates anti-inflammatory and neuroprotective effects in both lipopolysaccharide-(LPS)- and 1-methyl-4-phenylpyridinium-(MPP+)-mediated models of PD. For in vivo studies, a 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-(MPTP-) induced PD mouse model was used.
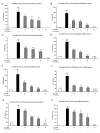
Results: FLZ showed potent efficacy in protecting dopaminergic (DA) neurons against LPS-induced neurotoxicity, as shown in rat and mouse primary mesencephalic neuronal-glial cultures by DA uptake and tyrosine hydroxylase (TH) immunohistochemical results. The neuroprotective effect of FLZ was attributed to a reduction in LPS-induced microglial production of proinflammatory factors such as superoxide, tumor necrosis factor-alpha (TNF-alpha), nitric oxide (NO) and prostaglandin E2 (PGE2). Mechanistic studies revealed that the anti-inflammatory properties of FLZ were mediated through inhibition of NADPH oxidase (PHOX), the key microglial superoxide-producing enzyme. A critical role for PHOX in FLZ-elicited neuroprotection was further supported by the findings that 1) FLZ's protective effect was reduced in cultures from PHOX-/- mice, and 2) FLZ inhibited LPS-induced translocation of the cytosolic subunit of p47PHOX to the membrane and thus inhibited the activation of PHOX. The neuroprotective effect of FLZ demonstrated in primary neuronal-glial cultures was further substantiated by an in vivo study, which showed that FLZ significantly protected against MPTP-induced DA neuronal loss, microglial activation and behavioral changes.
Conclusion: Taken together, our results clearly demonstrate that FLZ is effective in protecting against LPS- and MPTP-induced neurotoxicity, and the mechanism of this protection appears to be due, at least in part, to inhibition of PHOX activity and to prevention of microglial activation.
Figures
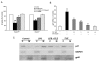

References
-
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

