Validation of syndromic surveillance for respiratory pathogen activity
- PMID: 18507902
- PMCID: PMC2600280
- DOI: 10.3201/eid1406.071467
Validation of syndromic surveillance for respiratory pathogen activity
Abstract
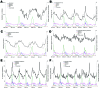
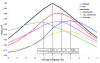
Syndromic surveillance is increasingly used to signal unusual illness events. To validate data-source selection, we retrospectively investigated the extent to which 6 respiratory syndromes (based on different medical registries) reflected respiratory pathogen activity. These syndromes showed higher levels in winter, which corresponded with higher laboratory counts of Streptococcus pneumoniae, respiratory syncytial virus, and influenza virus. Multiple linear regression models indicated that most syndrome variations (up to 86%) can be explained by counts of respiratory pathogens. Absenteeism and pharmacy syndromes might reflect nonrespiratory conditions as well. We also observed systematic syndrome elevations in the fall, which were unexplained by pathogen counts but likely reflected rhinovirus activity. Earliest syndrome elevations were observed in absenteeism data, followed by hospital data (+1 week), pharmacy/general practitioner consultations (+2 weeks), and deaths/laboratory submissions (test requests) (+3 weeks). We conclude that these syndromes can be used for respiratory syndromic surveillance, since they reflect patterns in respiratory pathogen activity.
Figures
References
-
- Fleming DM, Barley MA, Chapman RS. Surveillance of the bioterrorist threat: a primary care response. Commun Dis Public Health. 2004;7:68–72. - PubMed
-
- Miller M, Roche P, Spencer J, Deeble M. Evaluation of Australia’s National Notifiable Disease Surveillance System. Commun Dis Intell. 2004;28:311–23. - PubMed
-
- Ohkusa Y, Shigematsu M, Taniguchi K, Okabe N. Experimental surveillance using data on sales of over-the-counter medications—Japan, November 2003–April 2004. MMWR Morb Mortal Wkly Rep. 2005;54(Suppl):47–52. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
