Multifunctional magnetic nanoparticles for targeted imaging and therapy
- PMID: 18508157
- PMCID: PMC2583936
- DOI: 10.1016/j.addr.2008.03.014
Multifunctional magnetic nanoparticles for targeted imaging and therapy
Abstract
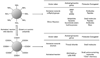
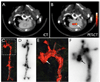
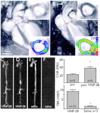
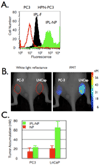
Magnetic nanoparticles have become important tools for the imaging of prevalent diseases, such as cancer, atherosclerosis, diabetes, and others. While first generation nanoparticles were fairly nonspecific, newer generations have been targeted to specific cell types and molecular targets via affinity ligands. Commonly, these ligands emerge from phage or small molecule screens, or are based on antibodies or aptamers. Secondary reporters and combined therapeutic molecules have further opened potential clinical applications of these materials. This review summarizes some of the recent biomedical applications of these newer magnetic nanomaterials.
Figures


References
-
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 65;2000:271–284. - PubMed
-
- Ohgushi M, Nagayama K, Wada A. Dextran-magnetite: A new relaxation reagent and its application to T2 measurements in gel systems. Journal of Magnetic Resonance (1969–1992) 1978;29:599–601.
-
- Halbreich A, Roger J, Pons JN, Geldwerth D, Da Silva MF, Roudier M, Bacri JC. Biomedical applications of maghemite ferrofluid. Biochimie. 1998;80:379–390. - PubMed
-
- Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanopartiocles in biomedicine. J Phys D: Appl Phys. 2003;36:R167–R181.
-
- Weissleder R, Stark DD, Compton CC, Wittenberg J, Ferrucci JT. Ferrite-enhanced MR imaging of hepatic lymphoma: An experimental study in rats. AJR Am J Roentgenol. 1987;149:1161–1165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

