The diverse functions of Src family kinases in macrophages
- PMID: 18508521
- PMCID: PMC4457449
- DOI: 10.2741/3015
The diverse functions of Src family kinases in macrophages
Abstract
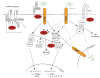
Macrophages are key components of the innate immune response. These cells possess a diverse repertoire of receptors that allow them to respond to a host of external stimuli including cytokines, chemokines, and pathogen-associated molecules. Signals resulting from these stimuli activate a number of macrophage functional responses such as adhesion, migration, phagocytosis, proliferation, survival, cytokine release and production of reactive oxygen and nitrogen species. The cytoplasmic tyrosine kinase Src and its family members (SFKs) have been implicated in many intracellular signaling pathways in macrophages, initiated by a diverse set of receptors ranging from integrins to Toll-like receptors. However, it has been difficult to implicate any given member of the family in any specific pathway. SFKs appear to have overlapping and complementary functions in many pathways. Perhaps the function of these enzymes is to modulate the overall intracellular signaling network in macrophages, rather than operating as exclusive signaling switches for defined pathways. In general, SFKs may function more like rheostats, influencing the amplitude of many pathways.
Figures

References
-
- Lowell CA. Src-family kinases: rheostats of immune cell signaling. Mol Immunol. 2004;41:631–43. - PubMed
-
- Miyazaki T, Tanaka S, Sanjay A, Baron R. The role of c-Src kinase in the regulation of osteoclast function. Mod Rheumatol. 2006;16:68–74. - PubMed
-
- Alvarez RH, Kantarjian HM, Cortes JE. The role of Src in solid and hematologic malignancies: development of new-generation Src inhibitors. Cancer. 2006;107:1918–29. - PubMed
-
- Chen T, George JA, Taylor CC. Src tyrosine kinase as a chemotherapeutic target: is there a clinical case? Anticancer Drugs. 2006;17:123–31. - PubMed
-
- Superti-Furga G, Gonfloni S. A crystal milestone: the structure of regulated Src. Bioessays. 1997;19:447–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
