Role of oxidative stress and nitric oxide in atherothrombosis
- PMID: 18508590
- PMCID: PMC2617738
- DOI: 10.2741/3084
Role of oxidative stress and nitric oxide in atherothrombosis
Abstract
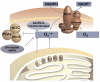
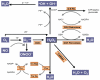
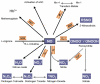
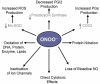
During the last decade basic and clinical research has highlighted the central role of reactive oxygen species (ROS) in cardiovascular disease. Enhanced production or attenuated degradation of ROS leads to oxidative stress, a process that affects endothelial and vascular function, and contributes to vascular disease. Nitric oxide (NO), a product of the normal endothelium, is a principal determinant of normal endothelial and vascular function. In states of inflammation, NO production by the vasculature increases considerably and, in conjunction with other ROS, contributes to oxidative stress. This review examines the role of oxidative stress and NO in mechanisms of endothelial and vascular dysfunction with an emphasis on atherothrombosis.
Figures
References
-
- Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. - PubMed
-
- Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol. 1994;266:H2568–H2572. - PubMed
-
- Meneshian A, Bulkley GB. The physiology of endothelial xanthine oxidase: from urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation. 2002;9:161–175. - PubMed
-
- Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
