Analysis of the membrane topology of transmembrane segments in the C-terminal hydrophobic domain of the yeast vacuolar ATPase subunit a (Vph1p) by chemical modification
- PMID: 18508769
- PMCID: PMC2475690
- DOI: 10.1074/jbc.M803258200
Analysis of the membrane topology of transmembrane segments in the C-terminal hydrophobic domain of the yeast vacuolar ATPase subunit a (Vph1p) by chemical modification
Abstract
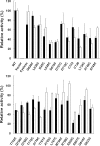
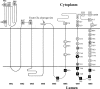
The integral V(0) domain of the vacuolar (H(+))-ATPases (V-ATPases) provides the pathway by which protons are transported across the membrane. Subunit a is a 100-kDa integral subunit of V(0) that plays an essential role in proton translocation. To better define the membrane topology of subunit a, unique cysteine residues were introduced into a Cys-less form of the yeast subunit a (Vph1p) and the accessibility of these cysteine residues to modification by the membrane permeant reagent N-ethylmaleimide (NEM) and the membrane impermeant reagent polyethyleneglycol maleimide (PEG-mal) in the presence and absence of the protein denaturant SDS was assessed. Thirty Vph1p mutants containing unique cysteine residues were constructed and analyzed. Cysteines introduced between residues 670 and 710 and between 807 and 840 were modified by PEG-mal in the absence of SDS, indicating a cytoplasmic orientation. Cysteines introduced between residues 602 and 620 and between residues 744 and 761 were modified by NEM but not PEG-mal in the absence of SDS, suggesting a lumenal orientation. Finally, cysteines introduced at residues 638, 645, 648, 723, 726, 734, and at nine positions between residue 766 and 804 were modified by NEM and PEG-mal only in the presence of SDS, consistent with their presence within the membrane or at a protein-protein interface. The results support an eight transmembrane helix (TM) model of subunit a in which the C terminus is located on the cytoplasmic side of the membrane and provide information on the location of hydrophilic loops separating TM6, 7, and 8.
Figures

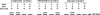

Similar articles
-
Definition of membrane topology and identification of residues important for transport in subunit a of the vacuolar ATPase.J Biol Chem. 2011 Oct 7;286(40):35176-86. doi: 10.1074/jbc.M111.273409. Epub 2011 Aug 8. J Biol Chem. 2011. PMID: 21832060 Free PMC article.
-
Structural analysis of the N-terminal domain of subunit a of the yeast vacuolar ATPase (V-ATPase) using accessibility of single cysteine substitutions to chemical modification.J Biol Chem. 2013 Aug 2;288(31):22798-808. doi: 10.1074/jbc.M113.460295. Epub 2013 Jun 5. J Biol Chem. 2013. PMID: 23740254 Free PMC article.
-
Transmembrane topography of the 100-kDa a subunit (Vph1p) of the yeast vacuolar proton-translocating ATPase.J Biol Chem. 1999 May 21;274(21):14655-61. doi: 10.1074/jbc.274.21.14655. J Biol Chem. 1999. PMID: 10329659
-
Structure and function of the vacuolar H+-ATPase: moving from low-resolution models to high-resolution structures.J Bioenerg Biomembr. 2003 Aug;35(4):337-45. doi: 10.1023/a:1025728915565. J Bioenerg Biomembr. 2003. PMID: 14635779 Review.
-
Subunit structure, function, and arrangement in the yeast and coated vesicle V-ATPases.J Bioenerg Biomembr. 2003 Aug;35(4):291-9. doi: 10.1023/a:1025720713747. J Bioenerg Biomembr. 2003. PMID: 14635775 Review.
Cited by
-
Ophthalmic phenotype of TCIRG1 gene mutations in Chinese infantile malignant osteopetrosis.BMJ Open Ophthalmol. 2018 Nov 17;3(1):e000180. doi: 10.1136/bmjophth-2018-000180. eCollection 2018. BMJ Open Ophthalmol. 2018. PMID: 30539151 Free PMC article.
-
Dissection of Protonation Sites for Antibacterial Recognition and Transport in QacA, a Multi-Drug Efflux Transporter.J Mol Biol. 2019 May 17;431(11):2163-2179. doi: 10.1016/j.jmb.2019.03.015. Epub 2019 Mar 23. J Mol Biol. 2019. PMID: 30910733 Free PMC article.
-
The Toxoplasma plant-like vacuolar compartment (PLVAC).J Eukaryot Microbiol. 2022 Nov;69(6):e12951. doi: 10.1111/jeu.12951. Epub 2022 Oct 27. J Eukaryot Microbiol. 2022. PMID: 36218001 Free PMC article. Review.
-
Autosomal recessive osteopetrosis: report of 41 novel mutations in the TCIRG1 gene and diagnostic implications.Osteoporos Int. 2012 Nov;23(11):2713-8. doi: 10.1007/s00198-011-1878-5. Epub 2012 Jan 10. Osteoporos Int. 2012. PMID: 22231430
-
C1 Silencing Attenuates Inflammation and Alveolar Bone Resorption in Endodontic Disease.J Endod. 2019 Jul;45(7):898-906. doi: 10.1016/j.joen.2019.02.024. Epub 2019 May 16. J Endod. 2019. PMID: 31104818 Free PMC article.
References
-
- Forgac, M. (2007) Nat. Rev. Mol. Cell. Biol. 8 917–929 - PubMed
-
- Nelson, N. (2003) J. Bioenerg. Biomembr. 35 281–289 - PubMed
-
- Gruenberg, J., and van der Goot, F. G. (2006) Nat. Rev. Mol. Cell. Biol. 7 495–504 - PubMed
-
- Wagner, C. A., Finberg, K. E., Breton, S., Marshansky, V., Brown, D., and Geibel, J. P. (2004) Physiol. Rev. 84 1263–1314 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

