Are three additional cycles of chemotherapy useful in patients with advanced-stage epithelial ovarian cancer after a complete response to six cycles of intravenous adjuvant paclitaxel and carboplatin?
- PMID: 18508785
- PMCID: PMC2435294
- DOI: 10.1093/jjco/hyn034
Are three additional cycles of chemotherapy useful in patients with advanced-stage epithelial ovarian cancer after a complete response to six cycles of intravenous adjuvant paclitaxel and carboplatin?
Abstract
Background: To evaluate the efficacy of three additional cycles of chemotherapy in patients with the International Federation of Gynecology and Obstetrics Stage III or IV, who achieved a complete response after six cycles of intravenous adjuvant paclitaxel/carboplatin after surgery.
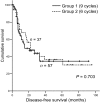
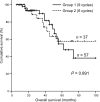
Methods: The clinical data of 94 patients with complete response after six cycles of adjuvant paclitaxel/carboplatin after surgery between January 1997 and March 2007 were reviewed retrospectively. Three additional cycles using the same chemotherapy were administered to 57 patients as consolidation chemotherapy (Group 1). Thirty-seven patients without the additional cycles served as controls (Group 2). Disease-free survival (DFS) and overall survival (OS) were evaluated using the Kaplan-Meier method with the log-rank test. The importance of consolidation chemotherapy as a prognostic factor affecting survival was examined using the Cox's proportional hazard analysis. The incidence of chemotherapy-induced hematological toxicities was compared between the two groups using chi-square test.
Results: Median DFS and mean OS were not significantly different between the two groups (15 versus 22 months, P = 0.703; 69 versus 73 months, P = 0.891, respectively). Consolidation chemotherapy was not a prognostic factor of survival although optimal debulking surgery and lower value of serum CA-125 levels after six cycles of the chemotherapy were prognostic factors improving DFS (P < 0.01). Grade 3 or 4 leukopenia was more common in patients treated with consolidation chemotherapy than in those not treated (50.9 versus 21.6%, P = 0.004).
Conclusion: Consolidation chemotherapy using paclitaxel/carboplatin may be inefficient and relatively toxic to advanced-stage epithelial ovarian cancer patients with complete response to six cycles of the same chemotherapy after surgery.
Figures
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. - PubMed
-
- Sugiyama T, Ushijima K, Kamura T. New regimens for the treatment of gynecologic cancers. Gan To Kagaku Ryoho. 2000;27:375–81. - PubMed
-
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. - PubMed
-
- Pecorelli S, Benedet JL, Creasman WT, Shepherd JH. FIGO staging of gynecologic cancer. 1994–1997 FIGO Committee on Gynecologic Oncology. International Federation of Gynecology and Obstetrics. Int J Gynaecol Obstet. 1999;65:243–9. - PubMed
-
- Berek J. Berek & Novak‘s gynecology. In: Berek JS, Natarajan S, editors. Ovarian and Fallopian Tube Cancer. 14th edn. Philadelphia: Lippincott Williams & Wilkins; 2007.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

