Regulation of the epithelial sodium channel by membrane trafficking
- PMID: 18508877
- PMCID: PMC2636908
- DOI: 10.1152/ajprenal.90248.2008
Regulation of the epithelial sodium channel by membrane trafficking
Abstract
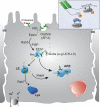
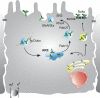
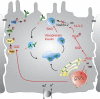
The epithelial Na(+) channel (ENaC) is a major regulator of salt and water reabsorption in a number of epithelial tissues. Abnormalities in ENaC function have been directly linked to several human disease states including Liddle's syndrome, psuedohypoaldosteronism, and cystic fibrosis and may be implicated in states as diverse as salt-sensitive hypertension, nephrosis, and pulmonary edema. ENaC activity in epithelial cells is highly regulated both by open probability and number of channels. Open probability is regulated by a number of factors, including proteolytic processing, while ENaC number is regulated by cellular trafficking. This review discusses current understanding of apical membrane delivery, cell surface stability, endocytosis, retrieval, and recycling of ENaC and the molecular partners that have so far been shown to participate in these processes. We review known sites and mechanisms of hormonal regulation of trafficking by aldosterone, vasopressin, and insulin. While many details of the regulation of ENaC trafficking remain to be elucidated, knowledge of these mechanisms may provide further insights into ENaC activity in normal and disease states.
Figures
References
-
- Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta 1695: 189–207, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

