Distribution, organization, and ecology of bacteria in chronic wounds
- PMID: 18508940
- PMCID: PMC2519454
- DOI: 10.1128/JCM.00501-08
Distribution, organization, and ecology of bacteria in chronic wounds
Abstract
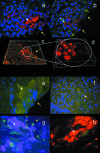
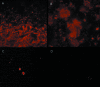
Between 1 and 2% of the population in the developed world experiences a nonhealing or chronic wound characterized by an apparent arrest in a stage dominated by inflammatory processes. Lately, research groups have proposed that bacteria might be involved in and contribute to the lack of healing of these wounds. To investigate this, we collected and examined samples from chronic wounds obtained from 22 different patients, all selected because of suspicion of Pseudomonas aeruginosa colonization. These wound samples were investigated by standard culturing methods and peptide nucleic acid-based fluorescence in situ hybridization (PNA FISH) for direct identification of bacteria. By means of the culturing methods, Staphylococcus aureus was detected in the majority of the wounds, whereas P. aeruginosa was observed less frequently. In contrast, using PNA FISH, we found that a large fraction of the wounds contained P. aeruginosa. Furthermore, PNA FISH revealed the structural organization of bacteria in the samples. It appeared that P. aeruginosa aggregated as microcolonies imbedded in the matrix component alginate, which is a characteristic hallmark of the biofilm mode of growth. The present investigation suggests that bacteria present within these wounds tend to be aggregated in microcolonies imbedded in a self-produced matrix, characteristic of the biofilm mode of growth. Additionally, we must conclude that there exists no good correlation between bacteria detected by standard culturing methods and those detected by direct detection methods such as PNA FISH. This strongly supports the development of new diagnostic and treatment strategies for chronic wounds.
Figures
References
-
- Bjarnsholt, T., P. O. Jensen, M. Burmolle, M. Hentzer, J. A. Haagensen, H. P. Hougen, H. Calum, K. G. Madsen, C. Moser, S. Molin, N. Hoiby, and M. Givskov. 2005. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 151373-383. - PubMed
-
- Bjarnsholt, T., P. O. Jensen, T. B. Rasmussen, L. Christophersen, H. Calum, M. Hentzer, H. P. Hougen, J. Rygaard, C. Moser, L. Eberl, N. Hoiby, and M. Givskov. 2005. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology 1513873-3880. - PubMed
-
- Bjarnsholt, T., K. Kirketerp-Moller, P. O. Jensen, K. G. Madsen, R. Phipps, K. Krogfelt, N. Hoiby, and M. Givskov. 2008. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 162-10. - PubMed
-
- Bjarnsholt, T., K. Kirketerp-Moller, S. Kristiansen, R. Phipps, A. K. Nielsen, P. O. Jensen, N. Hoiby, and M. Givskov. 2007. Silver against Pseudomonas aeruginosa biofilms. APMIS 115921-928. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

