Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after photobleaching
- PMID: 18508956
- PMCID: PMC2492615
- DOI: 10.1104/pp.108.120212
Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after photobleaching
Abstract
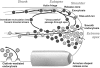
The delivery of cell wall material and membrane to growing plant cell surfaces requires the spatial and temporal coordination of secretory vesicle trafficking. Given the small size of vesicles, their dynamics is difficult to quantify. To quantitatively analyze vesicle dynamics in growing pollen tubes labeled with the styryl dye FM1-43, we applied spatiotemporal correlation spectroscopy on time-lapse series obtained with high-speed confocal laser scanning microscopy recordings. The resulting vector maps revealed that vesicles migrate toward the apex in the cell cortex and that they accumulate in an annulus-shaped region adjacent to the extreme tip and then turn back to flow rearward in the center of the tube. Fluorescence recovery after photobleaching confirmed vesicle accumulation in the shoulder of the apex, and it revealed that the extreme apex never recovers full fluorescence intensity. This is consistent with endocytotic activity occurring in this region. Fluorescence recovery after photobleaching analysis also allowed us to measure the turnover rate of the apical vesicle population, which was significantly more rapid than the theoretical rate computed based on requirements for new cell wall material. This may indicate that a significant portion of the vesicles delivered to the apex does not succeed in contacting the plasma membrane for delivery of their contents. Therefore, we propose that more than one passage into the apex may be needed for many vesicles before they fuse to the plasma membrane and deliver their contents.
Figures




Comment in
-
Not-so-tip-growth.Plant Signal Behav. 2009 Feb;4(2):136-8. doi: 10.4161/psb.4.2.7633. Plant Signal Behav. 2009. PMID: 19649191 Free PMC article.
References
-
- Baluška F, Liners F, Hlavacka A, Schlicht M, Van Cutsem P, McCurdy DW, Menzel D (2005) Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma 225 141–155 - PubMed
-
- Bartnicki-Garcia S (1990) Role of vesicles in apical growth and a new mathematical model of hyphal morphogenesis. In IB Heath, ed, Tip Growth in Plant and Fungal Cells. Academic Press, San Diego, pp 211–232
-
- Bartnicki-Garcia S (2002) Hyphal tip growth: outstanding questions. In HD Osiewacz, ed, Molecular Biology of Fungal Development. Marcel Dekker, New York, pp 29–58
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

