Characterization of the sodium/hydrogen exchanger NHA2
- PMID: 18508966
- PMCID: PMC2488271
- DOI: 10.1681/ASN.2007111245
Characterization of the sodium/hydrogen exchanger NHA2
Abstract
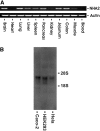
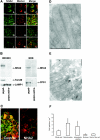
Cation/proton exchange has been recognized for decades in mammalian mitochondria, but the exchanger proteins have eluded identification. In this study, a cDNA from a human brain library, previously designated NHA2 in the genome, was cloned and characterized. The NHA2 transcript bears more similarity to prokaryotic than known eukaryotic sodium/proton exchangers, but it was found to be expressed in multiple mammalian organs and cultured cells. A mAb to NHA2 was generated and found to label an approximately 55-kD native protein in multiple tissues and cell lines. The specificity of this antibody was confirmed by demonstrating the loss of the native NHA2 band on immunoblots when cultured cells were treated with NHA2-specific small interfering RNA. Although NHA2 protein was detected in multiple organs, within each, its expression was restricted to specific cell types. In the kidney, co-localization with calbindin 28k and reverse transcription-PCR of microdissected tubules revealed that NHA2 is limited to the distal convoluted tubule. In cell lines, native NHA2 was localized both to the plasma membrane and to the intracellular compartment; immunogold electron microscopy of rat distal convoluted tubule demonstrated NHA2 predominantly but not exclusively on the inner mitochondrial membrane. Furthermore, co-sedimentation of NHA2 antigen and mitochondrial membranes was observed with differential centrifugation, and two mitochondrial markers co-localized with NHA2 in cultured cells. Regarding function, human NHA2 reversed the sodium/hydrogen exchanger-null phenotype when expressed in sodium/hydrogen exchanger-deficient yeast and restored the ability to defend high salinity in the presence of acidic extracellular pH. In summary, NHA2 is a ubiquitous mammalian sodium proton/exchanger that is restricted to the distal convoluted tubule in the kidney.
Figures





References
-
- Orlowski J, Grinstein S: Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch 447: 549–565, 2004 - PubMed
-
- Mitchell P, Moyle J: Stoichiometry of proton translocation through the respiratory chain and adenosine triphosphatase systems of rat liver mitochondria. Nature 208: 147–151, 1965 - PubMed
-
- Wang D, King SM, Quill TA, Doolittle LK, Garbers DL: A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol 5: 1117–1122, 2003 - PubMed
-
- Putney LK, Denker SP, Barber DL: The changing face of the Na+/H+ exchanger, NHE1: Structure, regulation, and cellular actions. Annu Rev Pharmacol Toxicol 42: 527–552, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

