Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress
- PMID: 18509042
- PMCID: PMC2796223
- DOI: 10.1523/JNEUROSCI.0552-08.2008
Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress
Abstract
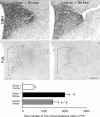
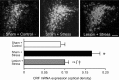
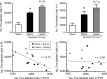
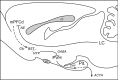
The medial prefrontal cortex (mPFC) has been proposed to play a role in the inhibition of hypothalamo-pituitary-adrenal (HPA) responses to emotional stress via influences on neuroendocrine effector mechanisms housed in the paraventricular hypothalamic nucleus (PVH). Previous work also suggests an involvement of the locus ceruleus (LC) in behavioral and neuroendocrine responses to a variety of acute stressors. The LC issues a widespread set of noradrenergic projections, and its innervation of the prefrontal cortex plays an important role in the modulation of working memory and attention. Because these operations are likely to be critical for stimulus selection, evaluation, and comparison with past experience in mounting adaptive responses to emotional stress, it follows that the LC-to-mPFC pathway might also be involved in regulating HPA activity under such conditions. Therefore, in the present study, we assessed the effects of selectively ablating noradrenergic inputs into the mPFC, using the axonally transported catecholamine immunotoxin, saporin-conjugated antiserum to dopamine-beta-hydroxylase, on acute restraint stress-induced activation of HPA output. Immunotoxin injections in the dorsal mPFC (centered in the prelimbic cortex) attenuated increments in restraint-induced Fos and corticotropin-releasing factor mRNA expression in the neurosecretory region of PVH, as well as HPA secretory responses. Stress-induced Fos expression in dorsal mPFC was enhanced after noradrenergic deafferentation and was negatively correlated with stress-induced PVH activation, independent of lesion status. These findings identify the LC as an upstream component of a circuitry providing for dorsal mPFC modulation of emotional stress-induced HPA activation.
Figures




Similar articles
-
Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress.J Neurosci. 2006 Dec 13;26(50):12967-76. doi: 10.1523/JNEUROSCI.4297-06.2006. J Neurosci. 2006. PMID: 17167086 Free PMC article.
-
A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response.J Neurosci. 2009 Jun 3;29(22):7330-40. doi: 10.1523/JNEUROSCI.5924-08.2009. J Neurosci. 2009. PMID: 19494154 Free PMC article.
-
Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience.Brain Res. 2007 Dec;1186:212-23. doi: 10.1016/j.brainres.2007.07.100. Epub 2007 Sep 19. Brain Res. 2007. PMID: 18001698 Free PMC article.
-
Reduced hypothalamo-pituitary-adrenal axis stress responses in late pregnancy: central opioid inhibition and noradrenergic mechanisms.Ann N Y Acad Sci. 2008 Dec;1148:428-38. doi: 10.1196/annals.1410.032. Ann N Y Acad Sci. 2008. PMID: 19120138 Review.
-
The organization of the stress system and its dysregulation in depressive illness.Mol Psychiatry. 2015 Feb;20(1):32-47. doi: 10.1038/mp.2014.163. Epub 2014 Dec 9. Mol Psychiatry. 2015. PMID: 25486982 Review.
Cited by
-
Chronic stress and brain plasticity: Mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders.Neurosci Biobehav Rev. 2015 Nov;58:79-91. doi: 10.1016/j.neubiorev.2015.06.018. Epub 2015 Jun 24. Neurosci Biobehav Rev. 2015. PMID: 26116544 Free PMC article. Review.
-
The neural basis of attentional alterations in prenatally protein malnourished rats.Cereb Cortex. 2021 Jan 1;31(1):497-512. doi: 10.1093/cercor/bhaa239. Cereb Cortex. 2021. PMID: 33099611 Free PMC article.
-
Corticosteroids: way upstream.Mol Brain. 2010 Jan 11;3:2. doi: 10.1186/1756-6606-3-2. Mol Brain. 2010. PMID: 20180948 Free PMC article. Review.
-
Different stress-related phenotypes of BALB/c mice from in-house or vendor: alterations of the sympathetic and HPA axis responsiveness.BMC Physiol. 2010 Mar 9;10:2. doi: 10.1186/1472-6793-10-2. BMC Physiol. 2010. PMID: 20214799 Free PMC article.
-
The role of medial prefrontal cortex projections to locus ceruleus in mediating the sex differences in behavior in mice with inflammatory pain.FASEB J. 2021 Jul;35(7):e21747. doi: 10.1096/fj.202100319RR. FASEB J. 2021. PMID: 34151467 Free PMC article.
References
-
- Abercrombie M. Estimation of nuclear populations from microtome populations sections. Anat Rec. 1949;94:239–247. - PubMed
-
- Antoni G. Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev. 1986;7:351–378. - PubMed
-
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Prog Brain Res. 1996;107:379–402. - PubMed
-
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res. 2000;126:165–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
