The h channel mediates location dependence and plasticity of intrinsic phase response in rat hippocampal neurons
- PMID: 18509046
- PMCID: PMC2612942
- DOI: 10.1523/JNEUROSCI.0835-08.2008
The h channel mediates location dependence and plasticity of intrinsic phase response in rat hippocampal neurons
Abstract
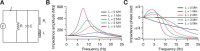
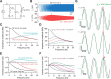
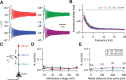
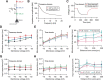
The presence of phenomenological inductances in neuronal membrane has been known for more than one-half a century. Despite this, the dramatic contributions of such inductive elements to the amplitude and, especially, phase of neuronal impedance, and their roles in modulating temporal dynamics of neuronal responses have surprisingly remained unexplored. In this study, we demonstrate that the h channel contributes a location-dependent and plastic phenomenological inductive component to the input impedance of CA1 pyramidal neurons. Specifically, we show that the h channels introduce an apparent negative delay in the local voltage response of these neurons with respect to the injected current within the theta frequency range. The frequency range and the extent of this lead expand with increases in h current either through hyperpolarization, or with increasing distance of dendritic location from the soma. We also demonstrate that a spatially widespread increase in this inductive phase component accompanies long-term potentiation. Finally, using impedance analysis, we show that both location and activity dependence of intrinsic phase response are attributable not to changes in a capacitive or a leak component, but to changes in h-channel properties. Our results suggest that certain voltage-gated ion channels can differentially regulate internal time delays within neurons, thus providing them with an independent control mechanism in temporal coding of neuronal information. Our analyses and results also establish impedance as a powerful measure of intrinsic dynamics and excitability, given that it quantifies temporal relationships among signals and excitability as functions of input frequency.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
