Cidea is associated with lipid droplets and insulin sensitivity in humans
- PMID: 18509062
- PMCID: PMC2409392
- DOI: 10.1073/pnas.0802063105
Cidea is associated with lipid droplets and insulin sensitivity in humans
Abstract
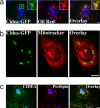
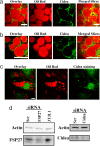
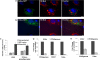
Storage of energy as triglyceride in large adipose-specific lipid droplets is a fundamental need in all mammals. Efficient sequestration of fat in adipocytes also prevents fatty acid overload in skeletal muscle and liver, which can impair insulin signaling. Here we report that the Cide domain-containing protein Cidea, previously thought to be a mitochondrial protein, colocalizes around lipid droplets with perilipin, a regulator of lipolysis. Cidea-GFP greatly enhances lipid droplet size when ectopically expressed in preadipocytes or COS cells. These results explain previous findings showing that depletion of Cidea with RNAi markedly elevates lipolysis in human adipocytes. Like perilipin, Cidea and the related lipid droplet protein Cidec/FSP27 are controlled by peroxisome proliferator-activated receptor gamma (PPARgamma). Treatment of lean or obese mice with the PPARgamma agonist rosiglitazone markedly up-regulates Cidea expression in white adipose tissue (WAT), increasing lipid deposition. Strikingly, in both omental and s.c. WAT from BMI-matched obese humans, expression of Cidea, Cidec/FSP27, and perilipin correlates positively with insulin sensitivity (HOMA-IR index). Thus, Cidea and other lipid droplet proteins define a novel, highly regulated pathway of triglyceride deposition in human WAT. The data support a model whereby failure of this pathway results in ectopic lipid accumulation, insulin resistance, and its associated comorbidities in humans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

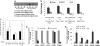
Similar articles
-
Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage.J Biol Chem. 2007 Nov 23;282(47):34213-8. doi: 10.1074/jbc.M707404200. Epub 2007 Sep 19. J Biol Chem. 2007. PMID: 17884815
-
Coordination Among Lipid Droplets, Peroxisomes, and Mitochondria Regulates Energy Expenditure Through the CIDE-ATGL-PPARα Pathway in Adipocytes.Diabetes. 2018 Oct;67(10):1935-1948. doi: 10.2337/db17-1452. Epub 2018 Jul 9. Diabetes. 2018. PMID: 29986925
-
Perilipin overexpression in white adipose tissue induces a brown fat-like phenotype.PLoS One. 2010 Nov 16;5(11):e14006. doi: 10.1371/journal.pone.0014006. PLoS One. 2010. PMID: 21103377 Free PMC article.
-
CIDE Family-Mediated Unique Lipid Droplet Morphology in White Adipose Tissue and Brown Adipose Tissue Determines the Adipocyte Energy Metabolism.J Atheroscler Thromb. 2017 Oct 1;24(10):989-998. doi: 10.5551/jat.RV17011. Epub 2017 Sep 5. J Atheroscler Thromb. 2017. PMID: 28883211 Free PMC article. Review.
-
Role of lipid droplet proteins in liver steatosis.J Physiol Biochem. 2011 Dec;67(4):629-36. doi: 10.1007/s13105-011-0110-6. Epub 2011 Aug 17. J Physiol Biochem. 2011. PMID: 21847662 Review.
Cited by
-
Fasting regulates FSP27 expression in the liver.J Lipid Res. 2013 Mar;54(3):569-570. doi: 10.1194/jlr.E036020. Epub 2013 Jan 18. J Lipid Res. 2013. PMID: 23335806 Free PMC article. No abstract available.
-
Transgenic Adipose-specific Expression of the Nuclear Receptor RORα Drives a Striking Shift in Fat Distribution and Impairs Glycemic Control.EBioMedicine. 2016 Sep;11:101-117. doi: 10.1016/j.ebiom.2016.08.027. Epub 2016 Aug 20. EBioMedicine. 2016. PMID: 27568222 Free PMC article.
-
Secondary Transcriptomic Analysis of Triple-Negative Breast Cancer Reveals Reliable Universal and Subtype-Specific Mechanistic Markers.Cancers (Basel). 2024 Oct 2;16(19):3379. doi: 10.3390/cancers16193379. Cancers (Basel). 2024. PMID: 39409999 Free PMC article.
-
Mechanism of Obesity-Related Lipotoxicity and Clinical Perspective.Adv Exp Med Biol. 2024;1460:131-166. doi: 10.1007/978-3-031-63657-8_5. Adv Exp Med Biol. 2024. PMID: 39287851 Review.
-
Metformin Inhibits Lipid Droplets Fusion and Growth via Reduction in Cidec and Its Regulatory Factors in Rat Adipose-Derived Stem Cells.Int J Mol Sci. 2022 May 26;23(11):5986. doi: 10.3390/ijms23115986. Int J Mol Sci. 2022. PMID: 35682666 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

