Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis
- PMID: 18509362
- PMCID: PMC4127033
- DOI: 10.1038/jid.2008.131
Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis
Abstract
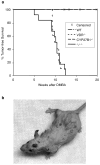
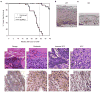
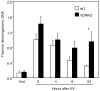
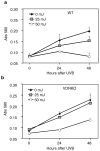
1,25-Dihydroxyvitamin D(3) (1,25(OH)(2)D(3)) is the biologically active ligand for the vitamin D receptor (VDR). VDR(-/-) mice have a hair follicle-cycling defect resulting in alopecia. However, mice lacking 25-hydroxyvitamin D(3) 1alpha-hydroxylase (CYP27B1(-/-)), and having no circulating 1,25(OH)(2)D(3), have normal follicular function. These mouse models indicate that VDR functions independently of 1,25(OH)(2)D(3) in regulating hair-follicle cycling. Here, we show that VDR(-/-) mice rapidly develop chemically induced skin tumors, whereas CYP27B1(-/-) and wild-type mice do not, indicating that VDR, and not the 1,25(OH)(2)D(3) ligand, is essential for protection against skin tumorigenesis. Because the majority of human skin cancer results from exposure to UV, the susceptibility of VDR(-/-) mice to this carcinogen was also evaluated. VDR(-/-) mice developed UV-induced tumors more rapidly and with greater penetrance than did VDR(+/+) mice. p53 protein levels were upregulated at similar rates in UV-treated keratinocytes of VDR(-/-) and VDR(+/+) mice. However, rates of thymine-dimer repair and UV-induced apoptosis were significantly lower in VDR(-/-) epidermis compared with the wild type epidermis. UV-induced epidermal thickening was also attenuated in VDR(-/-) skin, indicating that VDR plays a critical role in the repair and removal of severely damaged keratinocytes and adaptation of the skin to chronic UV exposure.
Conflict of interest statement
The authors state no conflict of interest.
Figures


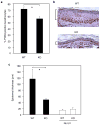
Comment in
-
Vitamin D receptor, UVR, and skin cancer: a potential protective mechanism.J Invest Dermatol. 2008 Oct;128(10):2357-61. doi: 10.1038/jid.2008.249. J Invest Dermatol. 2008. PMID: 18787544 Free PMC article.
References
-
- Albert MR, Weinstock MA. Keratinocyte carcinoma. CA Cancer J Clin. 2003;53:292–302. - PubMed
-
- Barletta F, Freedman LP, Christakos S. Enhancement of VDR-mediated transcription by phosphorylation: correlation with increased interaction between the VDR and DRIP205, a subunit of the VDR-interacting protein coactivator complex. Mol Endocrinol. 2002;16:301–314. - PubMed
-
- Bikle DD. Vitamin D and skin cancer. J Nutr. 2004;134:3472S–3478S. - PubMed
-
- Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, et al. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122:984–992. - PubMed
-
- Bikle DD, Nemanic MK, Whitney JO, Elias PW. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986;25:1545–1548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

