Sex ratio adjustment and kin discrimination in malaria parasites
- PMID: 18509435
- PMCID: PMC3807728
- DOI: 10.1038/nature06954
Sex ratio adjustment and kin discrimination in malaria parasites
Abstract
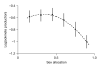
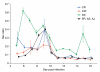
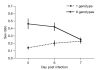
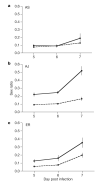
Malaria parasites and related Apicomplexans are the causative agents of the some of the most serious infectious diseases of humans, companion animals, livestock and wildlife. These parasites must undergo sexual reproduction to transmit from vertebrate hosts to vectors, and their sex ratios are consistently female-biased. Sex allocation theory, a cornerstone of evolutionary biology, is remarkably successful at explaining female-biased sex ratios in multicellular taxa, but has proved controversial when applied to malaria parasites. Here we show that, as predicted by theory, sex ratio is an important fitness-determining trait and Plasmodium chabaudi parasites adjust their sex allocation in response to the presence of unrelated conspecifics. This suggests that P. chabaudi parasites use kin discrimination to evaluate the genetic diversity of their infections, and they adjust their behaviour in response to environmental cues. Malaria parasites provide a novel way to test evolutionary theory, and support the generality and power of a darwinian approach.
Figures


Comment in
-
Evolutionary biology: sex ratios writ small.Nature. 2008 May 29;453(7195):605-6. doi: 10.1038/453605a. Nature. 2008. PMID: 18509433 No abstract available.
References
-
- Charnov EL. The Theory of Sex Allocation. Princeton Univ. Press; Princeton: 1982. - PubMed
-
- Frank SA. Sex allocation theory for birds and mammals. Annu. Rev. Ecol. Syst. 1990;21:13–55.
-
- Frank SA. A touchstone in the study of adaptation. Evolution Int. J. Org. Evolution. 2002;56:2561–2564.
-
- Hamilton WD. Extraordinary sex ratios. Science. 1967;156:477–488. - PubMed
-
- Hardy ICW. Sex Ratios: Concepts and Research Methods. Cambridge Univ. Press; Cambridge, UK: 2002.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

