Early neurodegeneration progresses independently of microglial activation by heparan sulfate in the brain of mucopolysaccharidosis IIIB mice
- PMID: 18509511
- PMCID: PMC2396504
- DOI: 10.1371/journal.pone.0002296
Early neurodegeneration progresses independently of microglial activation by heparan sulfate in the brain of mucopolysaccharidosis IIIB mice
Abstract
Background: In mucopolysaccharidosis type IIIB, a lysosomal storage disease causing early onset mental retardation in children, the production of abnormal oligosaccharidic fragments of heparan sulfate is associated with severe neuropathology and chronic brain inflammation. We addressed causative links between the biochemical, pathological and inflammatory disorders in a mouse model of this disease.
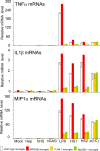
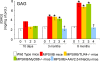
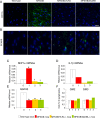
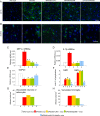
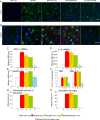
Methodology/principal findings: In cell culture, heparan sulfate oligosaccharides activated microglial cells by signaling through the Toll-like receptor 4 and the adaptor protein MyD88. CD11b positive microglial cells and three-fold increased expression of mRNAs coding for the chemokine MIP1alpha were observed at 10 days in the brain cortex of MPSIIIB mice, but not in MPSIIIB mice deleted for the expression of Toll-like receptor 4 or the adaptor protein MyD88, indicating early priming of microglial cells by heparan sulfate oligosaccharides in the MPSIIIB mouse brain. Whereas the onset of brain inflammation was delayed for several months in doubly mutant versus MPSIIIB mice, the onset of disease markers expression was unchanged, indicating similar progression of the neurodegenerative process in the absence of microglial cell priming by heparan sulfate oligosaccharides. In contrast to younger mice, inflammation in aged MPSIIIB mice was not affected by TLR4/MyD88 deficiency.
Conclusions/significance: These results indicate priming of microglia by HS oligosaccharides through the TLR4/MyD88 pathway. Although intrinsic to the disease, this phenomenon is not a major determinant of the neurodegenerative process. Inflammation may still contribute to neurodegeneration in late stages of the disease, albeit independent of TLR4/MyD88. The results support the view that neurodegeneration is primarily cell autonomous in this pediatric disease.
Conflict of interest statement
Figures
References
-
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. Faseb J. 2005;19:533–542. - PubMed
-
- Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, et al. Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson's disease. Faseb J. 2003;17:500–502. - PubMed
-
- Cunningham C, Deacon RM, Chan K, Boche D, Rawlins JN, et al. Neuropathologically distinct prion strains give rise to similar temporal profiles of behavioral deficits. Neurobiol Dis. 2005;18:258–269. - PubMed
-
- Moisse K, Strong MJ. Innate immunity in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762:1083–1093. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

