Calcineurin inhibitor withdrawal after renal transplantation with alemtuzumab: clinical outcomes and effect on T-regulatory cells
- PMID: 18510645
- PMCID: PMC11197848
- DOI: 10.1111/j.1600-6143.2008.02260.x
Calcineurin inhibitor withdrawal after renal transplantation with alemtuzumab: clinical outcomes and effect on T-regulatory cells
Abstract
To address the results of calcineurin inhibitor (CNI) withdrawal after alemtuzumab induction relative to CNI continuation, we performed a pilot randomized clinical trial in renal allograft recipients on CNI, a mycophenolic acid derivative and steroids after the first 2 months posttransplantation. Forty patients were randomized to taper off CNI or to maintain it, and followed for at least 1 year. Four patients in the withdrawal group were treated for acute rejection while no patient received antirejection treatment in the control group. Two control patients withdrew CNI due to nephrotoxicity. Estimated GFR was similar in both groups after 1 year. Flow cytometry of CD4(+)CD25(+)CTLA-4(+)FoxP3(+) regulatory T cells (Treg) demonstrated a significant increase in Treg percentages in the peripheral blood of alemtuzumab-treated patients on CNI early postransplant. Furthermore, the increased Treg percentages in the withdrawal cohort were unchanged at month 6 postenrollment, whereas they decreased significantly in those patients maintained on CNI. Patients withdrawn from CNI after alemtuzumab trend toward a higher rejection rate, but most patients can be weaned from a CNI using this regimen. With the exception of maintaining increased Treg levels, the benefits are not appreciable in this short follow-up, and a larger randomized trial is justified.
Figures
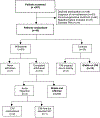

References
-
- 2006. Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1996–2005. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD; United Network for Organ Sharing, Richmond, VA.
-
- Magliocca JF, Knechtle SJ. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transplant Int 2006; 19: 705–714. - PubMed
-
- Knechtle SJ, Pirsch JD, Fechner JH et al. Campath-1Hinduction plus rapamycin monotherapy for renal transplantation: Results of a pilot study. Am J Transplant 2003; 3: 722–730. - PubMed
-
- Watson CJ, Bradley JA, Friend et al. Alemtuzumab (CAMPATH 1H) induction therapy in cadaveric kidney transplantation—efficacy and safety at five years. Am J Transplant 2005; 5: 1347–1353. - PubMed
-
- Knechtle SJ, Fernandez LA, Pirsch JD et al. Campath-1H in renal transplantation: The University of Wisconsin experience. Surgery 2004; 136: 754–760 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

