XPD helicase structures and activities: insights into the cancer and aging phenotypes from XPD mutations
- PMID: 18510924
- PMCID: PMC3055247
- DOI: 10.1016/j.cell.2008.04.030
XPD helicase structures and activities: insights into the cancer and aging phenotypes from XPD mutations
Abstract
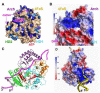
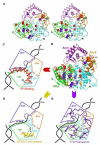
Mutations in XPD helicase, required for nucleotide excision repair (NER) as part of the transcription/repair complex TFIIH, cause three distinct phenotypes: cancer-prone xeroderma pigmentosum (XP), or aging disorders Cockayne syndrome (CS), and trichothiodystrophy (TTD). To clarify molecular differences underlying these diseases, we determined crystal structures of the XPD catalytic core from Sulfolobus acidocaldarius and measured mutant enzyme activities. Substrate-binding grooves separate adjacent Rad51/RecA-like helicase domains (HD1, HD2) and an arch formed by 4FeS and Arch domains. XP mutations map along the HD1 ATP-binding edge and HD2 DNA-binding channel and impair helicase activity essential for NER. XP/CS mutations both impair helicase activity and likely affect HD2 functional movement. TTD mutants lose or retain helicase activity but map to sites in all four domains expected to cause framework defects impacting TFIIH integrity. These results provide a foundation for understanding disease consequences of mutations in XPD and related 4Fe-4S helicases including FancJ.
Figures



References
-
- Bienstock RJ, Skorvaga M, Mandavilli BS, Van Houten B. Structural and functional characterization of the human DNA repair helicase XPD by comparative molecular modeling and site-directed mutagenesis of the bacterial repair protein UvrB. J. Biol. Chem. 2003;278:5309–5316. - PubMed
-
- Boal AK, Yavin E, Lukianova OA, O’Shea VL, David SS, Barton JK. DNA-bound redox activity of DNA repair glycosylases containing [4Fe-4S] clusters. Biochemistry. 2005;44:8397–8407. - PubMed
-
- Bootsma D, Hoeijmakers JH. DNA repair. Engagement with transcription. Nature. 1993;363:114–115. - PubMed
-
- Botta E, Nardo T, Lehmann AR, Egly JM, Pedrini AM, Stefanini M. Reduced level of the repair/transcription factor TFIIH in trichothiodystrophy. Hum Mol Genet. 2002;11:2919–2928. - PubMed
-
- Büttner K, Nehring S, Hopfner KP. Structural basis for DNA duplex separation by a superfamily-2 helicase. Nat. Struct. Mol. Biol. 2007;14:647–652. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

