The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates
- PMID: 18510965
- PMCID: PMC2842901
- DOI: 10.1016/j.jacc.2008.03.009
The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates
Abstract
Objectives: This study sought to develop a model that estimates the post-operative risk of right ventricular (RV) failure in left ventricular assist device (LVAD) candidates.
Background: Right ventricular failure after LVAD surgery is associated with increased morbidity and mortality, but identifying LVAD candidates at risk for RV failure remains difficult.
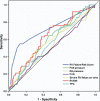
Methods: A prospectively collected LVAD database was evaluated for pre-operative clinical, laboratory, echocardiographic, and hemodynamic predictors of RV failure. Right ventricular failure was defined as the need for post-operative intravenous inotrope support for >14 days, inhaled nitric oxide for > or =48 h, right-sided circulatory support, or hospital discharge on an inotrope. An RV failure risk score (RVFRS) was created from multivariable logistic regression model coefficients, and a receiver-operating characteristic curve of the score was generated.
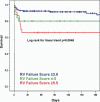
Results: Of 197 LVADs implanted, 68 (35%) were complicated by post-operative RV failure. A vasopressor requirement (4 points), aspartate aminotransferase > or =80 IU/l (2 points), bilirubin > or =2.0 mg/dl (2.5 points), and creatinine > or =2.3 mg/dl (3 points) were independent predictors of RV failure. The odds ratio for RV failure for patients with an RVFRS < or =3.0, 4.0 to 5.0, and > or =5.5 were 0.49 (95% confidence interval [CI] 0.37 to 0.64), 2.8 (95% CI 1.4 to 5.9), and 7.6 (95% CI 3.4 to 17.1), respectively, and 180-day survivals were 90 +/- 3%, 80 +/- 8%, and 66 +/- 9%, respectively (log rank for linear trend p = 0.0045). The area under the receiver-operating characteristic curve for the RVFRS (0.73 +/- 0.04) was superior to that of other commonly used predictors of RV failure (all p < 0.05).
Conclusions: The RVFRS, composed of routinely collected, noninvasive pre-operative clinical data, effectively stratifies the risk of RV failure and death after LVAD implantation.
Figures
References
-
- Dang NC, Topkara VK, Mercando M, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant. 2006;25:1–6. - PubMed
-
- Kavarana MN, Pessin-Minsley MS, Urtecho J, et al. Right ventricular dysfunction and organ failure in left ventricular assist device recipients: a continuing problem. Ann Thoracic Surg. 2002;73:745–50. - PubMed
-
- Deng MC, Edwards LB, Hertz MI, et al. Mechanical Circulatory Support Device Database of the International Society for Heart and Lung Transplantation: Third Annual Report—2005. J Heart Lung Transplant. 2005;24:1182–7. - PubMed
-
- Farrar DJ, Hill JD, Pennington DG, et al. Preoperative and postoperative comparison of patients with univentricular and biventricular support with the Thoratec ventricular assist device as a bridge to cardiac transplantation. J Thorac Cardiovasc Surg. 1997;113:202–9. - PubMed
-
- Fukamachi K, McCarthy PM, Smedira NG, et al. Preoperative risk factors for right ventricular failure after implantable left ventricular assist device insertion. Ann Thoracic Surg. 1999;68:2181–4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

