Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats
- PMID: 18511041
- PMCID: PMC2600773
- DOI: 10.1016/j.expneurol.2008.04.009
Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats
Abstract
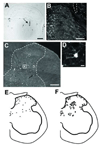
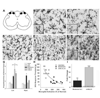
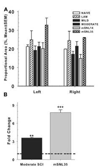
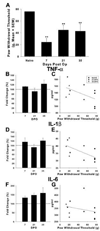
Spinal cord injury (SCI) impairs sensory systems causing chronic allodynia. Mechanisms underlying neuropathic pain have been more extensively studied following peripheral nerve injury (PNI) than after central trauma. Microglial activation, pro-inflammatory cytokine production and activation of p38 MAP kinase pathways may induce at-level allodynia following PNI. We investigated whether midthoracic SCI elicits similar behavioral and cellular responses below the level of injury (lumbar spinal cord; L5). Importantly, we show that anatomical connections between L5 and supraspinal centers remain intact after moderate SCI allowing direct comparison to a well-established model of peripheral nerve injury. We found that SCI elicits below-level allodynia of similar magnitude to at-level pain caused by a peripheral nerve injury. Moreover, the presence of robust microglial activation in L5 cord predicted allodynia in 86% of rats. Also increased phosphorylation of p38 MAP kinase occurred in the L5 dorsal horn of allodynic rats. For below-level allodynia after SCI, TNF-alpha and IL-1beta increased in the L5 dorsal horn by 7 dpo and returned to baseline by 35 dpo. Interestingly, IL-6 remains at normal levels early after SCI and increases at chronic time points. Increased levels of pro-inflammatory cytokines also occurred in the thalamus after SCI-induced allodynia. These data suggest that remote microglial activation is pivotal in the development and maintenance of below-level allodynia after SCI. Fractalkine, a known activator of microglia, and astrocytes were not primary modulators of below-level pain. Although the mechanisms of remote microglial activation are unknown, this response may be a viable target for limiting or preventing neuropathic pain after SCI in humans.
Figures


Comment in
-
Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury.Exp Neurol. 2008 Nov;214(1):6-9. doi: 10.1016/j.expneurol.2008.07.016. Epub 2008 Jul 29. Exp Neurol. 2008. PMID: 18708053 Free PMC article.
References
-
- International Association for the Study of Pain. Website 11-21-2005. 2008 Ref Type: Electronic Citation.
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J.Neurotrauma. 1995;12:1–21. - PubMed
-
- Behrmann DL, Bresnahan JC, Beattie MS, Shah BR. Spinal cord injury produced by consistent mechanical displacement of the cord in rats: Behavioral and histologic analysis. J.Neurotrauma. 1992;9:197–217. - PubMed
-
- Brenn D, Richter F, Schaible H-G. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: An inflammatory mechanism of joint pain. Arthritis Rheum. 2007;56:351–359. - PubMed
-
- Bruce JC, Oatway MA, Weaver LC. Chronic pain after clip-compression injury of the rat spinal cord. Exp.Neurol. 2002;178:33–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

