Neurobiology of cognitive aging: insights from imaging genetics
- PMID: 18511173
- PMCID: PMC3127547
- DOI: 10.1016/j.biopsycho.2008.03.015
Neurobiology of cognitive aging: insights from imaging genetics
Abstract
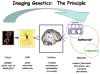
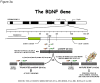
Over the last several years, neuroscientists have been increasingly using neuroimaging techniques to unravel the neurobiology underlying cognitive aging, and in more recent years to explore the role of genes on the variability of the aging process. One of the primary goals of this research is to identify proteins involved in cognitive aging with the hope that this would facilitate the development of novel treatments to combat cognitive impairment. Further, it is likely with early identification of susceptible individuals, early intervention through life-style changes and other methods could increase an individual's resilience to the effects of aging.
Figures





References
-
- Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, et al. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiol Aging. 2002;23(3):433–441. - PubMed
-
- Akyol O, Yanik M, Elyas H, Namli M, Canatan H, Akin H, et al. Association between Ala-9Val polymorphism of Mn-SOD gene and schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(1):123–131. - PubMed
-
- Bartres-Faz D, Junque C, Clemente IC, Lopez-Alomar A, Valveny N, Lopez-Guillen A, et al. Angiotensin I converting enzyme polymorphism in humans with age-associated memory impairment: relationship with cognitive performance. Neurosci Lett. 2000;290(3):177–180. - PubMed
-
- Baune BT, Hohoff C, Berger K, Neumann A, Mortensen S, Roehrs T, et al. Association of the COMT val158met variant with antidepressant treatment response in major depression. Neuropsychopharmacology. 2008;33(4):924–932. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

