Biomechanics of liquid-epithelium interactions in pulmonary airways
- PMID: 18511356
- PMCID: PMC2652855
- DOI: 10.1016/j.resp.2008.04.008
Biomechanics of liquid-epithelium interactions in pulmonary airways
Abstract
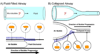
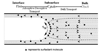
The delicate structure of the lung epithelium makes it susceptible to surface tension induced injury. For example, the cyclic reopening of collapsed and/or fluid-filled airways during the ventilation of injured lungs generates hydrodynamic forces that further damage the epithelium and exacerbate lung injury. The interactions responsible for epithelial injury during airway reopening are fundamentally multiscale, since air-liquid interfacial dynamics affect global lung mechanics, while surface tension forces operate at the molecular and cellular scales. This article will review the current state-of-knowledge regarding the effect of surface tension forces on (a) the mechanics of airway reopening and (b) epithelial cell injury. Due to the complex nature of the liquid-epithelium system, a combination of computational and experimental techniques are being used to elucidate the mechanisms of surface-tension induced lung injury. Continued research is leading to an integrated understanding of the biomechanical and biological interactions responsible for cellular injury during airway reopening. This information may lead to novel therapies that minimize ventilation induced lung injury.
Figures





References
-
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. - PubMed
-
- ARDS Clinical Network: The Assesment of Low Tidal Volume and Elevated End-expiratory Volume to Obviate Lung Injury (ALVEOLI) trial. 2004. vol. 2004 ( http://www.ardsnet.org/ards04.php).
-
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. - PubMed
-
- Anderson JC, Molthen RC, Dawson CA, Haworth ST, Bull JL, Glucksberg MR, Grotberg JB. Effect of ventilation rate on instilled surfactant distribution in the pulmonary airways of rats. Journal of Applied Physiology. 2004;97:45–56. - PubMed
-
- Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. A. M. A. Journal of Diseases of Children. 1959;97:517–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

